Physicomechanical and antimicrobial characteristics of hydrogel based on poly(vinyl alcohol): Performance improvement via inclusion of chitosan-modified nanoclay
Department of Chemical and Polymer Engineering, Faculty of Engineering, Yazd University, Yazd 891581-8411, Iran 2Department of Textile and Polymer Engineering, Islamic Azad University, Yazd Branch, Yazd 89165-1145, Iran 3School of Chemical Engineering, Co
Mehdi Entezam,1 Ashkan Ehghaghiyan,2 Negar Naghdi Sedeh,2 Seyed Hassan Jafari,3 Hossein Ali Khonakdar4,5
1 Abstract
The effect of a chitosan-modified nanoclay (CMNC) on the physical, mechanical, and antimicrobial properties of poly(vinyl alcohol) (PVA) hydrogels prepared by the electron beam irradiation method is reported in comparison with pristine nanoclay (PNC). The X-ray diffraction (XRD) results confirm that the chitosan modification process of nanoclay led to an enhancement in the clay gal- lery spacing. The inclusion of nanoclays in the PVA matrix decreased the gel content while it increased the swelling degree of the hydro- gels. Both PNC and CMNC played a role, depending on their amounts, in swelling of the hydrogel. The swelling kinetic studies revealed a diffusion-controlled swelling process. The diffusion coefficient of water molecules in hydrogels was decreased in the presence of PNC, while it increased with CMNC. Rheological investigations verified the influential role of nanoclays in decreasing the chemical crosslink density of the hydrogel. CMNC exhibited a higher reinforcing effect on hydrogel mechanical properties than PNC did, although the rhe- ological analysis, in agreement with the XRD results, indicated a better dispersion of PNC in the PVA matrix. According to the antimi- crobial tests, perfect inhibition of bacteria growth was obtained only for the hydrogels with CMNC.
2 Introduction
Hydrogels are hydrophilic polymeric networks that can absorb water into their cavities and interchain spaces. The use of poly- meric hydrogels as dressings is very important for the treatment of superficial and partial thickness burns.1 One of the methods for preparing hydrogels with the desired physical, mechanical, and chemical properties is to crosslink the polymer structure dur- ing the irradiation of an aqueous polymer solution using ionizing electrons and gamma rays. In this method, without using chemi- cal agents, it is also possible to sterilize the hydrogel.2
Among the various types of synthetic polymers, poly(vinyl alco- hol) (PVA) is very attractive from scientific and commercial viewpoints because of its biocompatibility, biodegradability, non- toxicity, noncarcinogenicity, water solubility, and in particular relatively low cost compared to most synthetic polymers.3,4 Onthe other hand, its shortcomings are poor mechanical properties and poor thermal stability. Studies have shown that the use of natural polymers along with nanomaterials can significantly improve these deficiencies.5–7 Among natural polymers, chitosan (CS) is used in conjunction with PVA because of its medical applications and features such as biocompatibility, biodegradabil- ity, antimicrobial properties, and nontoxicity.8–11 Also, incorpora- tion of nanofillers such as nanoclay can improve the performance of natural polymeric hydrogels.12–14 Chitosan, as a cationic bio- polymer, is also suitable for use as a modifier for clay in biologi- cal applications.15
Kokabi et al.16 prepared a nanocomposite hydrogel wound dressing based on PVA/nanoclay (Na-montmorillonite, MMT) by a freeze– thaw method. Their results showed that the dressing had favorable properties such as good swelling, increased moisture transfer rate, antimicrobial effect, and improved mechanical properties.
Kabiri and coworkers17,18 prepared poly-2-acrylamido-2-methyl- propane-1-sulfonic acid hydrogels containing chitosan-modified clay nanoparticles. It was shown that the use of modified clay improved the thermal stability, swelling behavior, and strength of the hydrogels.
Sirousazar et al.19 prepared PVA/Na-montmorillonite nanocom- posite hydrogels by the freeze–thaw method and studied the mor- phology, swelling behavior, and mechanical and thermal properties of the developed hydrogels. The results showed that increasing the nanoclay content improves the swelling behavior of the hydrogels and increases their mechanical properties and thermal stability.
Parparita et al.20 examined the properties of PVA/CS/nanoclay (Cloisite 30B) nanocomposites prepared by freezing and thawing under vacuum. It was shown that the addition of nanoclay to the hydrogel increased its thermal stability, mechanical properties, flexibility, and resistance to solvents. Noori et al.21 showed that the addition of MMT to a PVA/CS system improved the mechanical properties, biocompatibility, antibacterial activity, and swelling behavior of the nanocomposite hydrogels.
Mohsen et al.22 examined organic montmorillonite–poly(vinyl alcohol)-co-polyacrylic (OMMT-PVA/AAc) prepared by gamma radiation to absorb heavy metals from water. They reported that the presence of the OMMT increased the stability of the hydro- gels, while the gel content after the gamma exposure increased. This allowed the hydrogels to be reused several times.
The above-mentioned studies show that incorporation of nano- clay in PVA hydrogels in the presence of natural polymers such as CS could improve the mechanical properties, biocompatibility, biodegradation, and antimicrobial properties of the hydrogels. However, the extent of these improvements is limited by the lack of proper interactions between the hydrogel components. Also, a comparatively high content of CS, which is relatively expensive, is necessary to get their advantages in the hydrogels. It seems that modification of nanoclay by a natural polymer such as CS can overcome these limitations. To the best of our knowledge, reports on the modification of nanoclay by CS and its incorporation into PVA hydrogels are very rare. Therefore, the aim of this work is to investigate the effect of modification of nanoclay by chitosan, prepared via an electron-beam-assisted solution casting method, on the physical, mechanical, and antimicrobial properties of PVA hydrogels for wound dressing applications.
3 Experiment
3.1 Materials
PVA with a molecular weight of 72,000 g/mol and 98% hydroly- sis was supplied by Merck (Darmstadt, Germany). A low-molecu- lar-weight chitosan with a degree of acetic anhydride of 75%, K-10 montmorillonite nanoclay, and high-purity acetic acid sup- plied by Sigma-Aldrich (St. Louis, MO) were used.
3.2 Sample Preparation
Clay Modification. Clay nanoparticles were modified by chitosan according to an optimized method reported in the literature.23 Briefly, 4 g of nanoclay was dispersed in 50 mL of distilled water, and 1 g of chitosan was dissolved separately in 156 mL of aqueous 1% acetic acid solution, and then the two mixtures were blended, and the resulting mixture was sonicated for 30 min and then exposed to microwave radiation for 3 min. To remove excess chitosan, the mixture was centrifuged and washed with 1% acetic acid solution. Finally, the product was dried in an oven by air circulation at 60 ○C for 24 h.
3.3 Hydrogel Preparation.
PVA hydrogels were prepared from the PVA aqueous solution by electron beam irradiation. Initially, aque- ous solutions containing 12% (w/w) of PVA with various amounts (0, 1, 5, and 10 wt %) of modified or unmodified nanoclay (based on PVA weight) were prepared and mixed for 5 h at 90 ○C. In order to remove bubbles, the solutions were placed in an ultrasonic bath for 30 min at 80 ○C. Then the solutions were poured into polyethylene terephthalate molds with a thickness of 3 mm. After cooling the solution to ambient temperature, the solutions were covered by a polyethylene film impermeable against air and micro- organisms and then sealed in polyethylene bags. Finally, the molds containing the solution were irradiated by a high-energy electron beam at ambient temperature with a dose of 25 kGy. The electron beam irradiation was performed by an electron accelerator (Rhodotron TT200, Leuven, Belgium) with an energy of 10 MeV and a power of 80 kW. The dosimetry of the samples was per- formed using a triacetate cellulose film. The uncertainty in the dos- age measurement was about 4%, and the uniformity of the dose (ratio of maximum dose to minimum dose) was about 1.04.
3.4 X-ray Diffraction
The extent of nanoclay intercalation or exfoliation in the samples was studied by X-ray diffraction (XRD; Xpert, Philips, Amster- dam, Netherlands) using Cu Kα radiation (λ = 1.54 Å) at 40 kV and 40 mA in the region 2θ = 2○–10○ with a constant scan speed of 1○/min. The gallery spacing of the nanoclays was determined according to Bragg’s law as follows:
λ = 2d sin(θ)
where λ is the wavelength used in the test, D is distance between layers, and θ is the diffraction angle.
3.5 Gel Content
First, the hydrogel samples were dried in a vacuum oven at 60 ○C for 48 h. Then the samples were immersed in distilled water as solvent at 60 ○C. After the extraction cycle, the samples were again dried in a vacuum oven at 60 ○C to reach a constant weight. The gel content was obtained as follows:
Gel content(%)=Wd/Wi*100
where Wi and Wd are the initial weight and dry gel weight of the hydrogel, respectively.
3.6 Swelling Degree
Since one of the main features of hydrogels is their extent of absorption of water or physiological fluids in the body, changes in the swelling degree of hydrogels were determined. For this purpose, a specific amount of hydrogel was swelled in distilled water at 37 ○C. To determine the degree of equilibrium swelling, the hydrogel was taken out of the water, and after removing the excess surface water with a cotton cloth, it was weighed to a con- stant weight. The equilibrium swelling rate was determined according to the following relationship:
Degree of swelling(%)=(WS-Wi)/Wi*100
where Wi is the initial weight of the hydrogel, and Ws is the swelled gel weight. On average, three measurements for a given set of radiation conditions have been reported.
3.7 Rheological Analysis
The rheological behavior of hydrogels in an oscillating sinusoidal mode was investigated using an Anton Paar Physica Shear Rhe- ometer (MCR 300, Graz, Austria) with a parallel geometry of 25 mm diameter plates. The frequency sweeps were performed in the range 0.1–100 rad/s under air atmosphere at ambient temper- ature with a 0.2% strain.
3.8 Mechanical Properties
Tensile tests were carried out to determine the mechanical prop- erties of the samples using a HIWA 200 (Tehran, Iran) tensile machine according to ASTM D638. The measurements were car- ried out on samples with a thickness of 2 mm at ambient temper- ature and with a speed of 5 mm/min. An average of three measurements was reported for each sample.
3.9 Antimicrobial Analysis
In order to investigate the antibacterial activity of the hydrogels, shaking-flask and penetration tests were used. Escherichia coli (E. coli) and Staphylococcus aureus (S. aureus) bacteria were selected for the shaking-flask test. First, 0.5 mL of E. coli was mixed in a sample tube with 5 mL of a 0.01 M phosphate salt solution. Then, 0.2 g of each hydrogel sample was added to the solution. The tube was placed at 37 ○C for 1 h, and afterward 0.1 mL of the solution was placed on an agar medium on a plate. The plate was incubated at 37 ○C for 24 h. Finally, the number of colonies was counted, and the bacterial growth rate (GIB) was calculated as follows24:
GIB(%)=(A-B)/A*100
where A is the number of bacteria on the control plate, and B is the number of bacteria on the test plate.
Microbial penetration tests were done to determine the resistance of the hydrogel nanocomposite to microbial infiltration from the environment to the surface of the wound. A piece of each sample of hydrogel nanocomposite with a diameter of 5 cm and a thick- ness of 3 mm was placed in Tryptose Soy Agar (TSA) medium for 20 h at 30 ○C. Pseudomonas aeruginosa bacteria were trans- ferred to the hydrogel surface. The samples were then incubated at 30 ○C. The bacterial growth was monitored daily on the sur- face of the hydrogel in a TSA culture medium for one week.
4 RESULTS AND DISCUSSION
XRD patterns of the pristine and chitosan-modified clay are pre- sented in Figure 1. The pristine clay exhibits a diffraction peak at 2θ = 6.9○, equivalent to an interlayer spacing of 12.08 A, whereas this peak for the modified nanoclay appears at 2θ = 5.84○, corresponding to an interlayer spacing of 15.4 Å. The enhancement of the clay spacing implies that the chitosan chains have entered the clay layers, which in fact confirms the successful modification of the nanoclay by chitosan. These results are in agreement with the findings reported by Kabiri et al.23
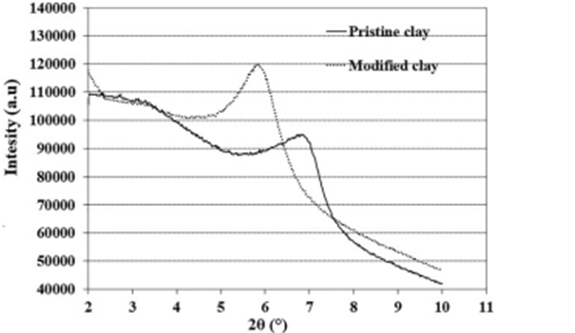
Figure 1. XRD patterns of unmodified and chitosan-modified nanoclays.
Figure 2 schematically shows the mechanism for modification of nanoclay by chitosan. In this mechanism, the amine group of chitosan is cationized in the acid solution and replaces the sodium cation existing between the clay layers through a cation exchange
process.
The XRD diagrams of PVA hydrogels containing modified and unmodified nanoclays are shown in Figure 3. The characteristic XRD peak for the pristine nanoclay dispersed in PVA hydrogel appears at 2θ = 3.6○, which is lower than the characteristic peak angle of the neat clay shown in Figure 1. This indicates that an intercalated structure has been formed in the hydrogels. The XRD results also show that the characteristic peak of the modi- fied nanoclay in the hydrogel appears at 2θ = 3.3○, which is lower than the peak angle of the pristine nanoclay in the hydrogel. In fact, the distance between the modified clay layers (26.74 Å) with the intercalated structure in the hydrogel matrix is larger than
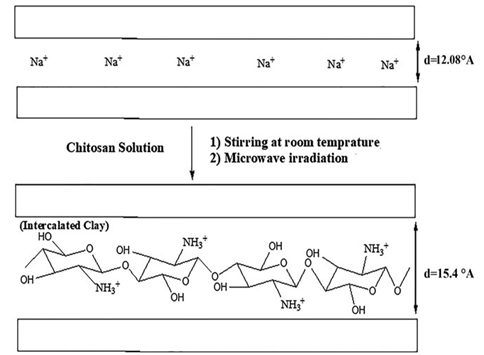
Figure 2. Schematic mechanism of nanoclay modification by chitosan.
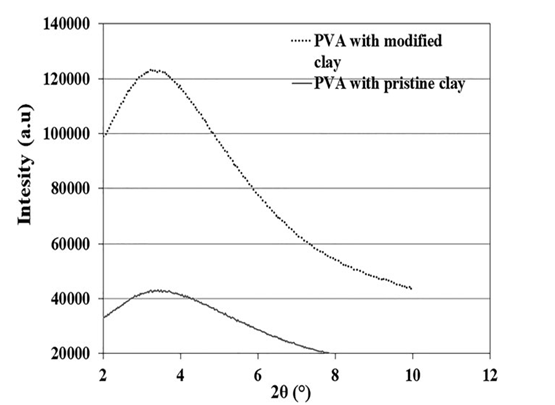
Figure 3. XRD patterns of PVA hydrogels loaded with 5 wt % of pristine or modified nanoclays.
that of the unmodified clay (24.51 Å). This observation is due to the simultaneous presence of chitosan and PVA chains in the interlayers of the modified nanoclay in the hydrogel. There is another remarkable difference between the XRD patterns of the unmodified and modified nanoclays in the hydrogel matrix. The peak intensity for unmodified nanoclay is much lower than that of the modified nanoclay, which is an indication of a better distri- bution of unmodified nanoclay in the hydrogel matrix as com- pared to the modified clay.
4.1 Gel Content and Swelling Behavior
The gel content results in terms of weight percent of nanoclay for hydrogels with modified and unmodified nanoclays are shown in Table I. These results clearly indicate that an increase in the nanoclay content of the hydrogels reduces the amount of gel. The observed gel reduction as a result of nanoclay inclusion has its roots in several factors, namely, reduction of active centers (radi- cals) on the chains, and reduction of the collision rate of these active centers, which in turns reduces the chances of formation of crosslinks. However, further studies are needed to make a more concrete conclusion. Another notable finding from the gel content results is that the gel content reduction of the hydrogel in the presence of the pristine clay is somewhat higher than that with the modified clay. This can be related to a better dispersion state of the pristine nanoclay in the hydrogel matrix, as con- firmed by the XRD results.
Table I. Gel Content of PVA Nanocomposite Hydrogels with Different Contents of Pristine or Modified Nanoclays
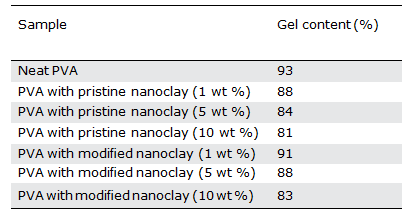
Table II. Equilibrium Swelling Degree of PVA Nanocomposite Hydrogels with Different Contents of Pristine or Modified Nanoclays
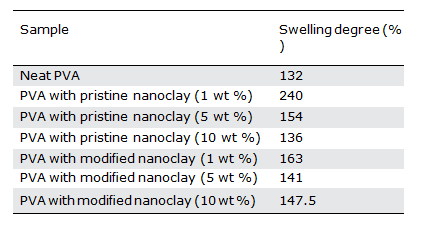
The equilibrium swelling degree, expressed as a weight percent of clay, for hydrogel samples containing modified or unmodified clays is shown in Table II. The results indicate that, in general, an increase in nanoclay content of the hydrogels increases their equilibrium swelling degree, which is advantageous for wound dressing usage. However, a closer look at the data reveals that the swelling degree of hydrogels initially increases and then decreases when increasing the nanoclay content beyond a certain level. The swelling phenomenon of hydrogels consists of three stages25:
- Diffusion of solvent molecules into polymer networks
- Release of polymer chains
- Expansion of the polymer network toward the outer medium of solvent
Considering the fact that the inclusion of nanoclay in the hydrogel reduces the gel content and consequently decreases the crosslink density of the hydrogels, it should facilitate all three pseudopheno- mena involved in the swelling process of hydrogels, and as a result its effect on increasing the swelling degree was predictable.
On the other hand, nanoclay can act as a physical barrier against solvent diffusion, and this hinders all three phenomena involved in the swelling process of the hydrogel. In fact, nanoclay plays two opposing roles in the swelling process of a hydrogel. Obviously both of these roles depend on the nanoclay content and its distribu- tion in the PVA matrix. At high loadings, the negative effect of nanoclay on the swelling process becomes more prominent.
Comparing the swelling degree of hydrogels containing modified nanoclay with the ones loaded with the unmodified nanoclay at a constant nanoclay content (Table II), we see that at low clay con- tents, like 1 wt %, the swelling rate of the samples containing unmodified nanoclay is much higher than that of the modified one. The differences become less at the higher loadings of nanoclay.
Link density of hydrogels containing unmodified nanoclay is lower than in the system with the modified clay. This results in higher swelling rates, especially at the low clay contents. However, based on the XRD results (Figure 3), the unmodified nanoclay is better distributed in the hydrogels, which results in a higher barrier effect against water penetration and the eventual reduced swelling degree. This effect seems to be more pronounced for the case of unmodi- fied nanoclay and becomes more effective at high clay loadings. This leads to the reduced differences in the rate of swelling of those two types of nanocomposite hydrogels at the high clay loadings.
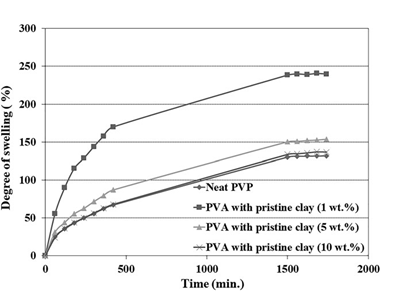
Figure 4. Variation of swelling degree against time for the PVA hydrogels containing various contents of pristine nanoclay.
Finally, it is important to note that chitosan itself as a modifier of nanoclay may have a role in reducing the hydrophilicity of the nanocomposite hydrogels containing the modified clays and thereby reduces their swelling degree.
4.2 Swelling Kinetics
In order to study the kinetics and mechanism of hydrogel swelling, the swelling degree of the hydrogels was measured at specific inter- vals of 1 h. Figure 4 shows time-dependent swelling diagrams for the neat PVA hydrogel and the PVA nanocomposite hydrogels contain- ing unmodified nanoclay. A similar set of diagrams for nanocompo- site hydrogels containing modified nanoclay is shown in Figure 5.
A comparison between these two set of diagrams indicates that the trends of changes in the swelling rates at any specific time for different nanoclay loadings are roughly the same as the ones observed for the equilibrium swelling degree of the hydrogels pre- sented in the previous section. Also, the results indicate that the final swelling is reached after about 30 h for most of the samples.
The Peppas model is one of the most common models for evalu- ating the kinetics and mechanism of hydrogel swelling26–28:
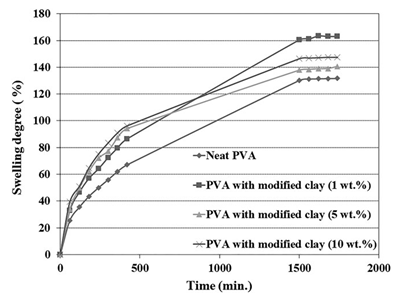
Figure 5. Variation of swelling degree against time for the PVA hydrogels containing various contents of chitosan-modified nanoclay.
Mt/M∞=ktn
where Mt/M∞ is the partial swelling at time t, k is a constant characteristic of the hydrogel system, and n is the diffusion expo[1]nent characteristic of the hydrogel swelling mechanism. The values n = 0.5, 0.5 < n < 1, and n = 1 indicate normal Fickian, non-Fickian, and type II diffusion mechanisms, respectively.26,27 The initial diffusion coefficient of water during the hydrogel swelling process can be calculated as follows26,27:
Mt/M∞=4(Dt/πL2)0.5
where D is the diffusion coefficient and L is the sample thickness. According to the literature, the value of Mt/M∞ is below 0.6.27,29
The values of n, k, and D for nanocomposite hydrogels contain- ing different contents of modified or unmodified nanoclays are presented in Table III.
Similar to the neat PVA hydrogel, the n values for all of the nano- composite hydrogels are about 0.5. An n value of 0.5 indicates that the swelling mechanism for all of the samples obeys the normal Fick- ian behavior from the initial to the middle times of the experiment.
The diffusion coefficient (D) values for all of the nanocomposite hydrogels containing modified nanoclay are higher than that of the neat PVA, which shows an increase in diffusion coefficient by increasing the modified nanoclay content in the hydrogel (Table III). The diffusion coefficient of water molecules into the hydrogel is strongly influenced by the density of crosslinks (chemi- cal and physical), as well as the barrier effect of the nanoclay on the hydrogel matrix. The lower the crosslink density of the hydro- gel, the higher the water molecular-diffusion coefficient into the hydrogel. On the other hand, the higher the amount of nanoclay content in the hydrogel matrix, the better their distribution in the hydrogel and their interaction with the hydrogel chains, which leads to a decrease in the diffusion coefficient of water molecules into the hydrogel. According to the results of the diffusion.
Table III. Swelling Kinetics Parameters from the Peppas Model for PVA Nano[1]composite Hydrogels with Different Contents of Pristine or Modified Nanoclays
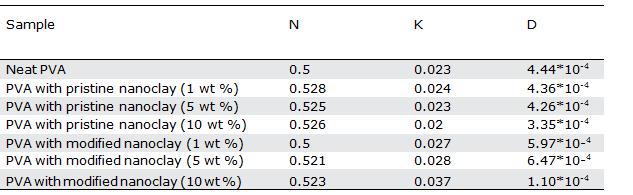
coefficient presented in Table III, it seems that reducing the density of chemical crosslinks in the hydrogel by the presence of modified nanoclay is the dominant effect for increasing the diffusion coeffi- cient of water molecules into the hydrogel.
Unlike the effect of modified nanoclay, the diffusion of water mol- ecules in PVA hydrogels decreases in the presence of the unmodi- fied nanoclay, and that is exaggerated by increasing the nanoclay content. It seems the negative effect of the unmodified nanoclay on the barrier properties and formation of physical crosslinks domi- nates its positive effect in reducing the chemical crosslinks, and therefore the diffusion coefficient of water molecules in the PVA matrix decreases in the presence of the unmodified nanoclay.
By comparing the opposing effects of these two types of nano- clays on the diffusion coefficient of water molecules in PVA hydrogels and the fact that the unmodified nanoclay has a much stronger negative effect, it can be deduced that, in agreement with the XRD results, the unmodified nanoclay has a better dispersion state in the PVA matrix than the modified one does.
4.3 Rheology
Figures 6(a,b) and 7(a,b) show the storage modulus and complex viscosity curves for nanocomposite hydrogels containing different contents of modified or unmodified nanoclays. A solid-like rheo- logical behavior is observed for all of the hydrogel samples, in agreement with their crosslinked structure.
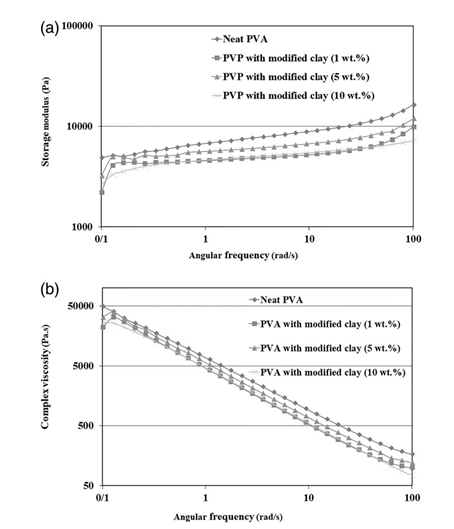
Figure 6. Storage modulus (a) and complex viscosity (b) as a function of frequency for the hydrogels loaded with various amounts of chitosan- modified nanoclay.
From Figure 6 it is seen that the presence of the modified nano- clay in the PVA hydrogel leads to deterioration of its rheological properties, especially its storage modulus [Figure 6(a)]. This is mainly due to a reduction in the density of chemical crosslinks, as confirmed by the gel content results (Table I). Also, the results reveal that the intensity of the reduction of the rheological prop- erties of nanocomposite hydrogels is strongly dependent on the nanoclay content of the hydrogels. The highest values of the rhe- ological properties are obtained for the sample with 5 wt % of modified nanoclay, while the samples with 1 and 10 wt % nano- clay exhibit the lowest values (Figure 6). In fact, two of the most influential factors affecting the rheological properties of nano- composite hydrogels are (1) the chemical and physical crosslink densities and (2) the physical crosslink strength. These factors themselves are affected by the amount and the dispersion state of the nanoclay in the PVA hydrogel. The higher the nanoclay con- tent and the better the nanoclay dispersion state, the lower the chemical crosslink density and the higher the physical crosslink density. Therefore, it appears that the lowest values of the rheo- logical properties for the nanocomposite hydrogels with 10 wt % modified nanoclay originate from the lowest chemical crosslink density and the worst dispersion state of the modified nanoclay.
According to Figure 7, the rheological properties of PVA hydrogels with 1 and 5 wt % pristine nanoclay are the same as for the hydro- gels with similar contents of modified nanoclay. However, the
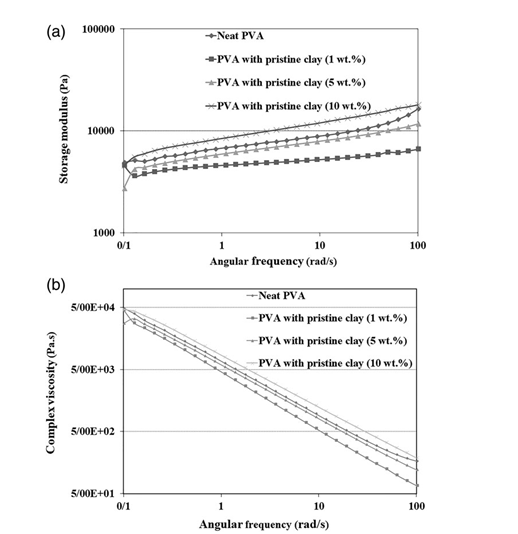
Figure 7. Storage modulus (a) and complex viscosity (b) as a function of frequency for the hydrogels loaded with various amounts of pristine nanoclay.
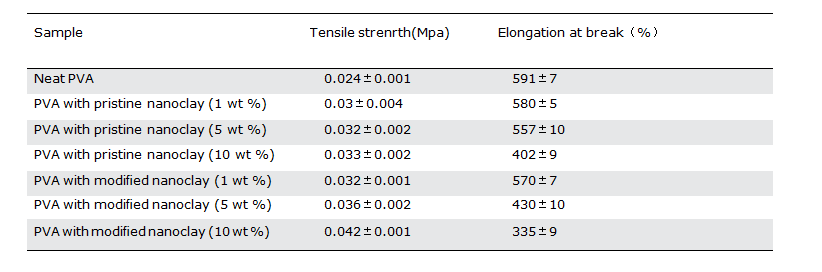
Table IV. Tensile Mechanical Properties of PVA Nanocomposite Hydro- gels with Different Contents of Pristine or Modified Nanoclay
hydrogel with 10 wt % pristine nanoclay shows higher values of rhe- ological properties than the neat PVA hydrogel. This implies that the physical crosslink density of the hydrogel nanocomposite sample with 10 wt % pristine nanoclay is higher than that of the system with 10 wt % modified nanoclay. This might be due to the good disper- sion state of the pristine nanoclay in the hydrogel, as verified by the XRD results (Figure 2). Moreover, the chemical crosslink density of the hydrogels loaded with pristine nanoclay is somewhat lower than that of the ones with modified nanoclay (Table I). It should be noted that, besides the chemical and physical crosslinks due to the PVA crystalline phase, the rheological properties of the nanocomposite hydrogel systems are also affected by the physical crosslinks originat- ing from interactions between PVA chains and clay particles. It seems that these physical crosslinks in the PVA nanocomposite hydrogel system loaded with 10 wt % pristine nanoclay, which has a better state of nanoclay dispersion than the system loaded with 10 wt% modified nanoclay, can compensate for the negative effects of the nanoclay on the reduction in chemical crosslinks and crystallinity of PVA. Therefore, the rheological properties of the PVA nanocompo- site hydrogel system loaded with 10 wt % of the pristine nanoclay are somewhat higher than that of the neat PVA hydrogel (Figure 7).
4.4 Mechanical Tensile Properties
The data for tensile strength and elongation at break for PVA hydrogels containing various contents of modified or unmodified nanoclays are summarized in Table IV. Based on these results, it is clear that the presence of nanoclay in the PVA matrix increases the tensile strength while slightly decreasing the elongation atbreak. These changes in the mechanical properties of hydrogels are enhanced by increasing the nanoclay content in the hydrogel. The reason for the observed changes in the mechanical propertiesof hydrogels with the presence of nanoclays has its roots in the interactions between the PVA chains and the nanoclays. These interactions play the role of physical crosslinks in the hydrogel and hence increase its tensile strength. On the other hand, due to the hindering effect of nanoclay on PVA chain mobility, the elon- gation at break of the nanocomposite hydrogels decreases slightly. By comparing the effect of similar loadings of modified and unmodified nanoclays on the mechanical properties of PVA hydrogels, it can be deduced that the modified nanoclay has a stronger effect on the mechanical properties of PVA hydrogels than the unmodified clay. Three reasons can be mentioned for these observations. The first one is the somewhat higher chemical crosslink density of the PVA hydrogels loaded with the modified nanoclay, and the second is the presence of chitosan as a modifier with higher mechanical properties. The third is the stronger interactions between the PVA chains and the modified nanoclay.
The different effects observed of the nanoclay type on the mechanical properties (Table IV) with respect to the rheological properties (Figures 6 and 7) can be attributed to the differences in the applied strains in these two tests. The rheological proper- ties are normally measured at very low strains (less than 0.2%), at which both the strong and weak interactions are highly influen- tial on the rheological properties, whereas mechanical properties such as tensile strength and elongation at break are the properties that are related to a large deformation, and therefore they are evaluated at relatively high strains. At such high strains, the strong interactions between polymer chains and nanoclay govern the mechanical properties of the system. In this context, the observed higher mechanical properties of the nanocomposites loaded with modified clay as compared to the ones with pristine nanoclays can be attributed to the stronger interactions existing in the former system, while the higher rheological properties of the latter system measured at low strains can be assigned to the better state of clay dispersion and more physical interactions between polymer chains and nanoclay particles. Such physical interactions lose their importance at large deformations.
4.5 Bacteria Count and Diffusion
In order to evaluate the antibacterial properties of the hydrogels, bacterial counting and diffusion tests were performed, and the results are presented in Table V. The results show that the PVA hydrogels containing the chitosan-modified nanoclay completely inhibited the growth of gram-negative and gram-positive bacteria, while the neat PVA hydrogel and the hydrogels loaded with the unmodified nanoclay only prevent about 30% of the bacteria growth. The better antibacterial characteristics of the PVA hydro- gel containing chitosan-modified nanoclay are related to the inherent antibacterial properties of chitosan.
Daily examinations of PVA hydrogels with a thickness of 3 mm in TSA medium showed that the Pseudomonas aeruginosa bacte- ria did not pass through either the neat hydrogel or any of the nanocomposite hydrogels, and therefore they can satisfactorily protect wounds from infection by germs. According to previous studies, a high number of layers of traditional gauze dressings cannot prevent foreign bacteria from entering the wound.30 How- ever, the PVA/nanoclay hydrogel nanocomposites developed in this work, especially the ones loaded with the chitosan-modified nanoclay, can be considered as effective wound dressings
5 CONCLUSIONS
In order to efficiently apply the advantages of chitosan along with the benefits of the nanoclay particles for PVA hydrogels as wound dress- ing, the nanoclays were modified by chitosan chains and applied to develop PVA nanocomposite hydrogels via an electron-beam-assisted solution casting method. In comparison with pristine nanoclay (PNC), the quantitative effect of the chitosan-modified nanoclay (CMNC) on the physicomechanical and antibacterial properties of the hydrogel was studied. The observed enhancement in nanoclay gallery spacing verified by XRD confirmed the successful modification pro- cess of nanoclay by chitosan. Moreover, the XRD and rheological investigations on the nanocomposite hydrogels revealed a better dis- persion of PNC in the PVA matrix as compared to the modified nano- clay. In agreement with the results for gel content, the rheological studies confirmed the reduction in chemical crosslink density of the hydrogels as a result of nanoclay inclusion. The swelling results showed that the nanocomposite hydrogels had higher swelling degree than the neat PVA hydrogel. Depending on the nanoclay content, the nanoclay had a dual effect on the swelling process of hydrogels. Analy- sis of the swelling kinetics by the Peppas model revealed that all of the hydrogels obeyed a normal Fickian diffusion mechanism. The diffu- sion coefficient of water molecules in nanocomposite hydrogels was decreased with increasing content of PNC, whereas an opposite trend was observed for the hydrogels loaded with CMNC. CMNC had a stronger effect on increasing the tensile strength of the hydrogels than PNC did. Antibacterial properties and inhibition of bacterial growth were only observed in the hydrogels loaded with the chitosan-modified nanoclay. Finally, incorporation of chitosan-modified nanoclay into the PVA hydrogels is found to be a promising method for developing wound dressings with suitable antibacterial properties and good mechanical properties, so PVA/CMNC nanocomposite hydrogels containing a comparatively low content of chitosan as a nanoclay modifier could be considered as an appropriate and economical alter- native to PVA/chitosan/clay and PVA/chitosan hydrogels.
6 REFERENCES
- Liptak, M. J. J. Vet. 1997, 75, 408.
- Ulanski, P.; Janik, I.; Kadlubowski, S.; Kozicki, M.; Kujawa, P.; Pietrzak, M.; Stasica, P.; Rosiak, J. M. J. Pol Polym. Adv. Technol. 2002, 13, 951.
- Valenta, C.; Anver, B. G. Eur. J. Pharm. Biopharm. 2004,58, 279.
- Entezam, M.; Daneshian, H.; Nasirizadeh, N.; Khonakdar, H. A.; Jafari, S. H. Macromol. Mater. Eng. 2017, 302, 1.
- Lyons, J. G.; Geever, L. M.; Nugent, M. J. D.; Kennedy, J. E.; Higginbotham, C. L. J. Mech. Behav. Biomed. Mater. 2009, 5, 485.
- Kamoun, E. A.; Chen, X.; Eldin, M. S. M.; Kenawy, E.-R. S. Arabian J. Chem. 2015, 8, 1.
- Yang, X.; Zhu, Z.; Liu, Q.; Chen, X.; Mingwang, M. Radiat. Phys. Chem. 2008, 77, 954.
- Varshney, L. Nucl. Instrum. Methods Phys. Res., Sect. B. 2007, 255, 343.
- Ranjha, N. M.; Khan, S. J. Pharm. Alt. Med. 2013, 2, 30.
- Vrana, E. N.; Liu, Y.; McGuinness, B. G.; Cahill, A. P. Macromol. Symp. J. 2008, 269, 106.
- Yang, W.; Fortunati, E.; Bertoglio, F.; Owczarek, J. S.; Brunif, G.; Kozanecki, M.; Kenny, J. M.; Torre, L.; Visai, L.; Puglia, D. Carbohydr. Polym. 2018, 181, 275.
- Lin, J.; Xu, S.; Shi, X.; Feng, S.; Wang, J. Polym. Adv. Tech- nol. 2009, 20, 645.
- Akpomie, K. G.; Dawodu, F. A. Beni Suef Univ. J. Basic. Appl. Sci. 2016, 5, 1.
- Feng, Y. L.; Wang, C.; Mao, N. Adv. Mater. J. 2017, 6, 20.
- Darder, M.; Colilla, M.; Ruiz-Hitzky, E. Chem. Mater. J. 2003, 15, 3774.
- Kokabi, M.; Sirousazar, M.; Hassan, M. Z. Eur. Polym. J. 2007, 43, 773.
- Kabiri, K.; Mirzadeh, H.; Zohuriaan-Mehr, M. J.; Daliri, M. Soc Chem. Ind. J. 2009, 58, 1252.
- Kabiri, K.; Mirzadeh, H.; Zohuriaan-Mehr, M. J. J. Appl. Polym. Sci. 2010, 116, 2548.
- Sirousazar, M.; Kokabi, K.; Hassan, M. Z.; Baharamian, A. R. J. Macromol. Sci., Part B: Phys. 2012, 51, 1335.
- Parpariţa, E.; Cheaburu, N. C.; Paţachia, F. S.; Vasile, C. Acta Chem. Iasi. 2014, 22, 75.
- Noori, S.; Kokabi, M.; Hassan, M. Z. Procedia Mater. Sci. 2015, 11, 152.
- Mohsen, M.; Gomaa, E.; Mazaid, A. N.; Mohammed, R. AIMS Mater. Sci. 2017, 4, 1122.
- Kabiri, K.; Mirzadeh, H.; Zohuriaan-Mehr, M. J. Iran. Polym. J. 2007, 16, 147.
- Maier, R. M.; Pepper, I. L.; Gerba, C. P.; Maier, R. Environ- mental Microbiology; Elsevier: Amsterdam, 2008. p. 22.
- Yoshida, R.; Okuyama, Y.; Sakai, K.; Okanoa, T.; Sakurai, Y. J. Membr. Sci. J. 1994, 89, 267.
- 26. Singh, B. Int. J. Pharm. 2007, 334, 1.
- Ritger, P. L.; Peppas, N. A. A. J. Controlled Release. 1987, 5, 23.
- Lee, P. I. J. Controlled Release. 1985, 2, 277.
- Yoshida, R.; Kaneko, Y.; Sakai, K.; Okanoa, T.; Sakurai, Y.; Bae, H. Y.; Kim, S. W. J. Controlled Release. 1994, 32, 97.
- 30. Purna, S. K.; Babu, M. Burns. 2000, 26, 54.