Review of the oral toxicity of polyvinyl alcohol (PVA)
Colorcon, 415 Moyer Boulevard, West Point, PA 19486, USA Accepted26 September 2002
Summary
Polyvinyl alcohols (PVA) (CAS no. 9002-89-5) are synthetic polymers used in a wide range of industrial, commercial, medical andfoodapplications. The purpose of this review, this critical evaluation of the available information on PVA, is to support the safety of PVA as a coating agent for pharmaceutical and dietary supplement products. All the available information on PVA gleaned from a comprehensive search of the scientific literature were critically evaluated. Orally administered PVA is relatively harmless. The safety of PVA is basedon the following: (1) the acute oral toxicity of PVA is very low, with LD50s in the range of 15–20 g/kg; (2) orally administered PVA is very poorly absorbed from the gastrointestinal tract; (3) PVA does not accumulate in the body when administered orally; (4) PVA is not mutagenic or clastogenic; and (5) NOAELs of orally administered PVA in male and female rats were 5000 mg/kg body weight/day in the 90-day dietary study and 5000 mg/kg body weight/day in the two-generation reproduction study, which was the highest dose tested. A critical evaluation of the existing information on PVA supports its safety for use as a coating agent for pharmaceutical and dietary supplement products.
# 2002 Elsevier Science Ltd. All rights reserved.
Keywords: Polyvinyl alcohol; Poly(vinyl alcohol); Partially hydrolyzed polyvinyl alcohol; PVA; Film coating polymer; Excipient; Vinyl alcohol polymer; Ethenol homopolymer
Introduction
Polyvinyl alcohols (PVA) are synthetic polymers used since the early 1930s in a wide range of industrial, com- mercial, medical and food applications including resins, lacquers, surgical threads and food-contact applica- tions. General chemical and physical properties of PVA are summarized in Table 1.
The physical characteristics of PVA are dependent on its method of preparation from the hydrolysis, or partial hydrolysis, of polyvinyl acetate (Fig. 1). PVA is gen- erally classified into two groups, partially hydrolyzed (A) and fully hydrolyzed (B).
Varying the length of the initial vinyl acetate polymer and the degree of hydrolysis under alkaline or acidic conditions yields PVA products of differing molecular weights (20,000–400,000), solubility, flexibility, tensile strength and adhesiveness. Various properties are mea- sured to characterize PVA such as pH, viscosity, loss on(Abbreviations: GMPs; good manufacturing practices; HNEL; highest-no-effect level; LEL; lowest-effect level; NOAEL; no-observed- adverse-effect level; PVA; polyvinyl alcohols.* Corresponding author.E-mail address: cdemerlis@colorcon.com (C.C. DeMerlis0278-6915/03/$ - see front matter # 2002 Elsevier Science Ltd. All rights reserved. PII: S0278 -6915 (02)00258 -2 )) drying, melting point, refractive index, heavy metals and residue on ignition. These properties vary based on molecular weight and% hydrolysis for the grade of PVA.
PVA is included in the Handbook of Pharmaceutical Excipients. Specifications for pharmaceutical use are provided in Japanese Pharmaceutical Excipients, United States Pharmacopeia/National Formulary and the Eur- opean Pharmacopoeia. Pharmaceutical grade PVA must be manufactured under CGMP standards. The phar- maceutical grades also require lower impurities and residual solvent levels then the industrial grades.
In the USA, the majority of PVA is used in the textile industries as a sizing and finishing agent. PVA can also be incorporated into a water-soluble fabric in the man- ufacture of degradable protective apparel, laundry bags for hospitals, rags, sponges, sheets, covers, as well as physiological hygiene products.
PVA is also widely used in the manufacture of paper products. As with textiles, PVA is applied as a sizing and coating agent. It provides stiffness to these products making it useful in tube winding, carton sealing and board lamination. PVA is used as a thickening agent for latex paint and common household white glue or in other adhesive mixtures such as remoistenable labels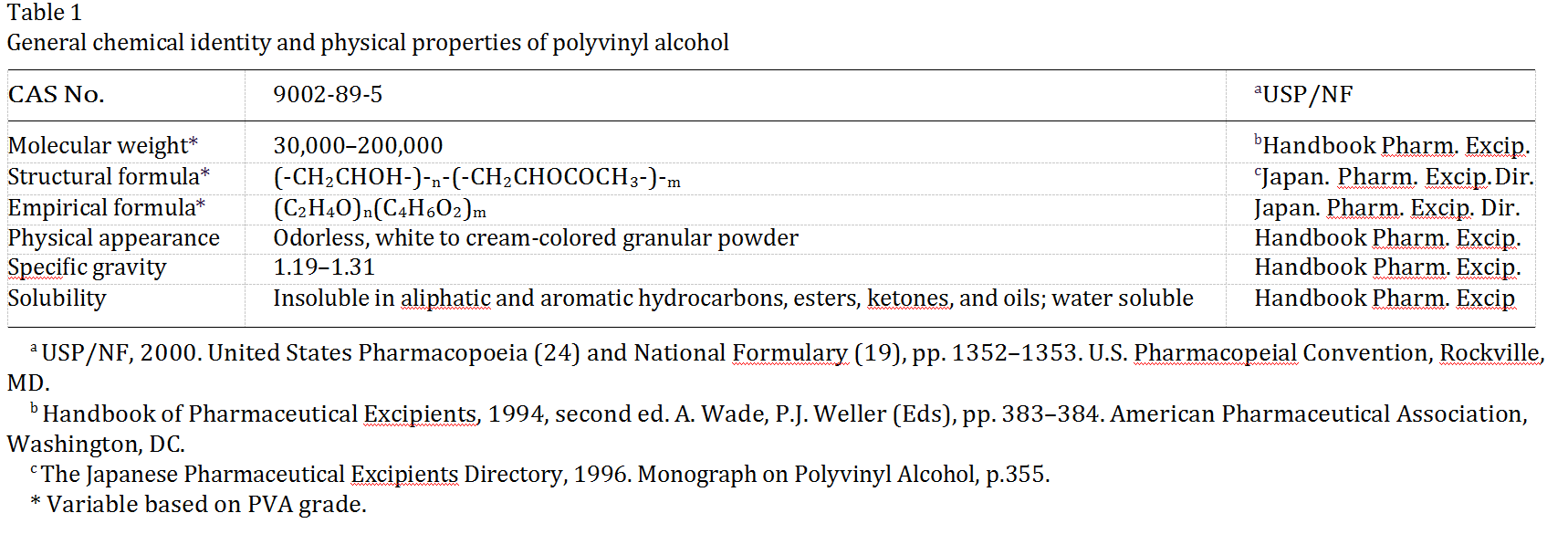
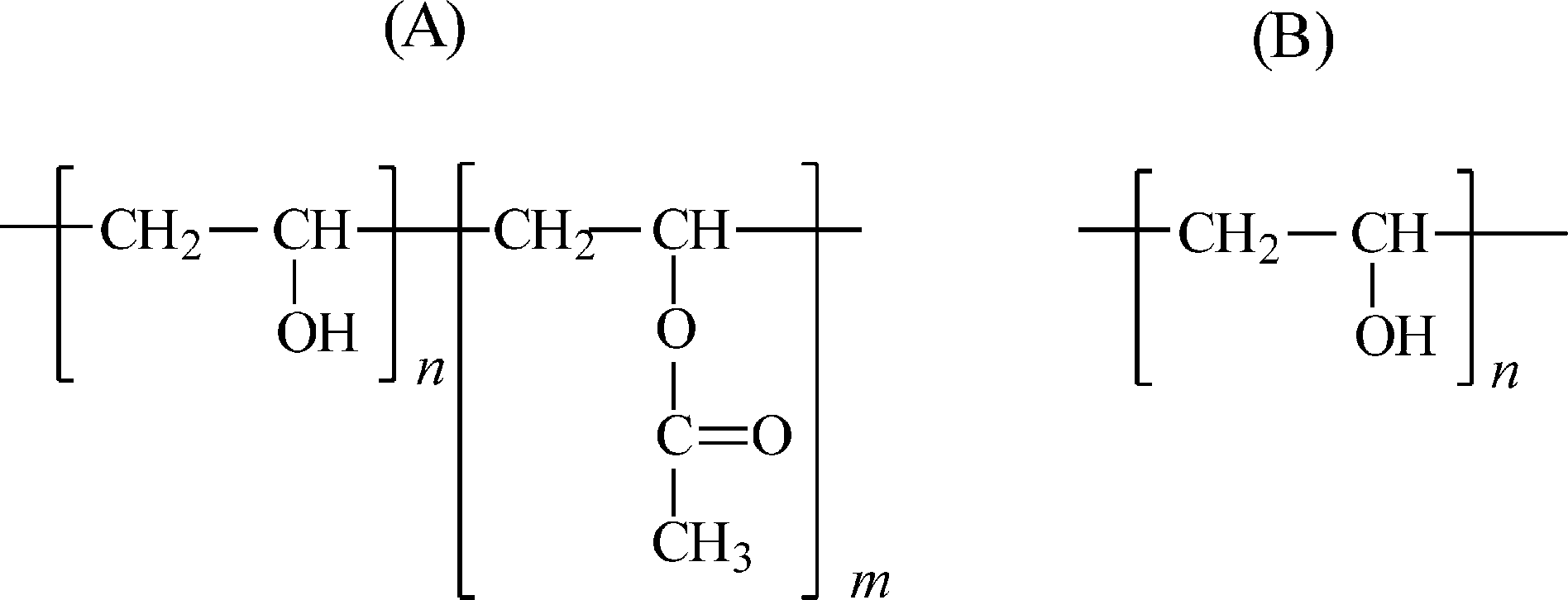 (Fig. 1. Structural formula for PVA: (A) partially hydrolyzed; (B) fully hydrolyzed.)
(Fig. 1. Structural formula for PVA: (A) partially hydrolyzed; (B) fully hydrolyzed.)
and seals, as well as gypsum-based cements such as is used for ceramic tiles. PVA is relatively insoluble in organic solvents and its solubility in aqueous solutions is adaptable to its necessary application (Isolyser, 1998). The US Food and Drug Administration allows PVA for use as an indirect food additive in products which are in contact with food (Table 2).
Under 21 CFR 73.1 (b) (2), PVA is approved as a diluent in color additive mixtures for coloring shell eggs. Under 21 CFR 349.12, PVA is approved as an oph- thalmic demulcent at 0.1–4.0%.
PVA has also been approved for use in packaging meat products by the Meat Inspection Division of the USDA and approved for use in packaging poultry pro- ducts by the Poultry Division of the USDA.
PVA is approved for use in several medical applica- tions including transdermal patches, the preparation of jellies that dry rapidly when applied to the skin and in immediate and sustained release tablet formulations. Cross-linked polyvinyl alcohol microspheres are also used for controlled release of oral drugs. Ophthalmic solutions, such as synthetic tears, may also contain PVA because it provides good dispersion and coating prop- erties. (Wade and Weller, 1994). PVA is included in the FDA Inactive Ingredient Guide for ophthalmic pre- parations and oral tablets. PVA will be evaluated for its food use by the Joint FAO/WHO Expert Committee on Food Additives (JECFA) in June 2003.
As an industrial and commercial product, PVA is valued for its solubility and biodegradability, which contributes to its very low environmental impact. Sev- eral microorganisms ubiquitous to artificial and natural environments—such as septic systems, landfills, com- post and soil—have been identified which are able to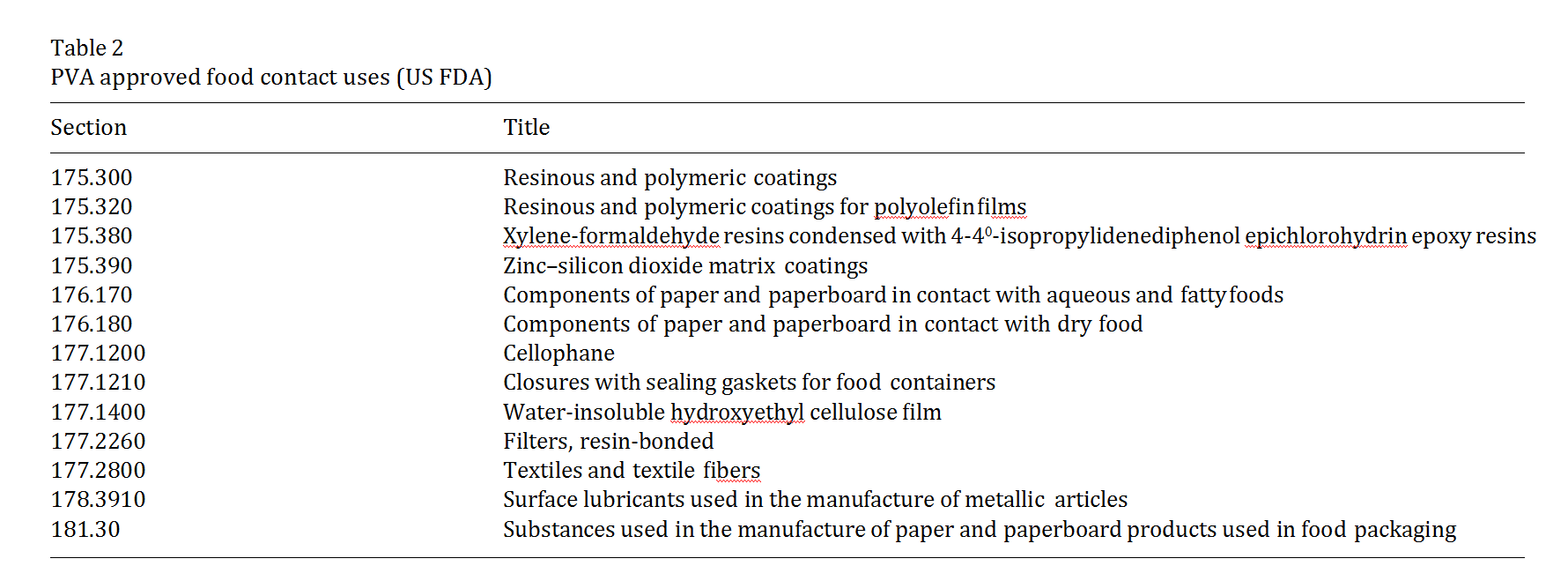 degrade PVA through enzymatic processes. A combi- nation of oxidase and hydrolase enzyme activities degrade PVA into acetic acid but both the percent hydrolysis and its solubility affect the rate of PVA bio- degradation (Isolyser, 1998).
degrade PVA through enzymatic processes. A combi- nation of oxidase and hydrolase enzyme activities degrade PVA into acetic acid but both the percent hydrolysis and its solubility affect the rate of PVA bio- degradation (Isolyser, 1998).
There are additional potential food uses for PVA currently under evaluation, including uses in con- fectionery products and high-moisture food products.
The purpose of this review, this critical evaluation of the available information on PVA, is to support the safety of PVA as a coating agent for pharmaceutical and dietary supplement products.
Toxicological data for PVA administered by routes other than the oral route are included in Appendix A.
Biological data
Absorption, distribution and excretion
The absorption, distribution and excretion of PVA (mol. wt <50,0001) was studied in male and female Fischer 344 rats (Sanders and Matthews, 1990). Greater than 98% of a single oral dose of 0.01 mg/kg 14C-label- led PVA administered to three male rats was recovered in the feces within 48 h of administration. Less than 0.2% of the total dose was detected in the urine. No PVA-derived radioactivity was detected as expired 14CO2 or volatiles. There was no detectable tissue accu- mulation. These data indicate that very little PVA is absorbed from the gastrointestinal tract.
To further assess possible bioaccumulation, 0.1 mg/kg body weight/day 14C-labelled PVA was administered by gavage daily for 10 consecutive days to three additional rats. Results similar to the initial study were found, almost all radioactivity was recovered in the feces, 0.2% of the total dose in the urine and 0.05% of the total dose detected in the major tissues (blood liver, kidneys, skin, muscle and adipose tissue) (Sanders and Mat- thews, 1990).
Toxicity studies
Acute toxicity
The results of acute toxicity studies are summarized in the Table 3. These data indicate that orally administered PVA would be placed in the category of least concern,.‘‘relatively harmless’’ (Hodge and Sterner, 1949).
Many studies did not report the molecular weight or the percent hydrolysis of PVA.
Repeated dosing (subacute) toxicity
Four rats were fed 2 g PVA mixed in 45 g of feed ( 2220 mg/kg body weight) for 2 weeks, followed by an additional 2 weeks at twice the concentration ( 4440 mg/kg body weight). Two of the rats were sacrificed at the end of the 4 weeks and the remaining rats were fed 10 g PVA mixed in 25 g of diet ( 20,000 mg/kg body weight) for an additional 2 weeks. The animals gained weight during the course of the study and no gross changes were observed at necropsy. The only micro- scopic findings at the highest dose were hepatic hydro- pic degeneration and eosinophilic infiltration of the submucosa of the stomach and the dense myeloid mar- row of the sternum (Hueper, 1939).
Ten mice received 500 mg PVA/kg body weight/day orally for 20 consecutive days; there were no deaths and no adverse effects reported (JSCI, 1968).
Subchronic toxicity
Thirty mice received daily oral doses of 100, 500 or 1000 mg/kg body weight/ day for 26 weeks. There were no treatment-related adverse effects (JSCI, 1968).
The highest-no-effect level (HNEL) in a 20-day dog study was 10,000 mg/kg body weight (Clydesdale, 1997b). The lowest-effect level (LEL) in a 180-day dog study was 800 mg/kg body weight. Emesis and diarrhea were noted (Clydesdale, 1997c).
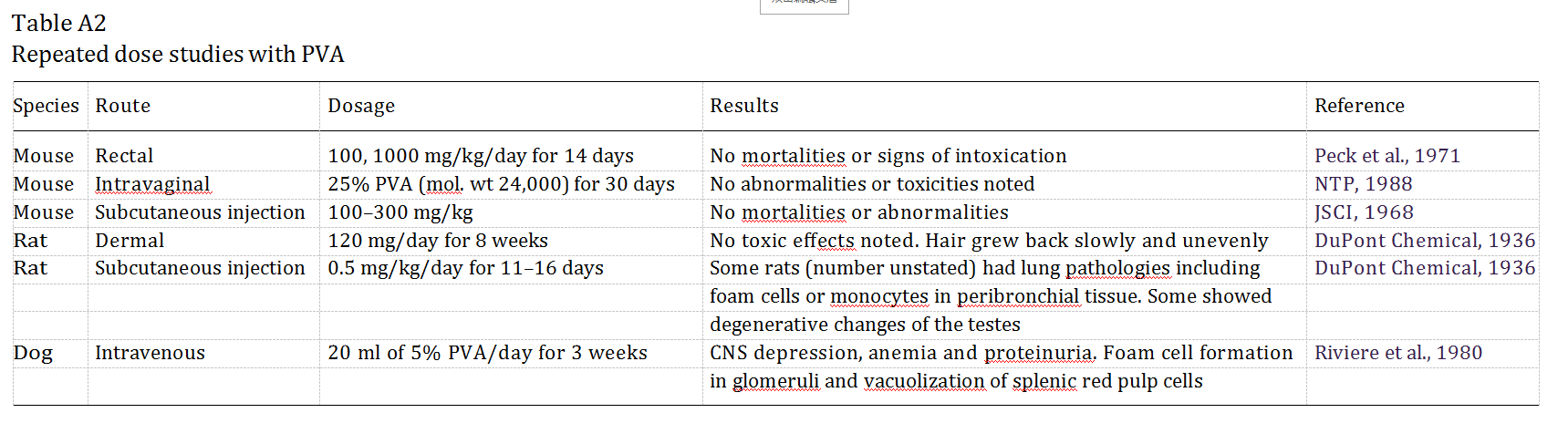
Repeated dose studies are listed in Table 4.
A special grade of PVA was used in new studies con- ducted by Colorcon that is produced to meet global pharmaceutical standards using good manufacturing practices (GMPs). The PVA grade is characterized by compendial and proprietary specifications. The impu- rities are strictly monitored by process controls, strin- gent specifications and analytical methods developed by the manufacturer.
Colorcon has conducted a specific subchronic toxicity study to provide current supportive information on the specific grade of PVA used as a coating agent for phar- maceutical and dietary supplement products, as well as its potential food use. In this study, PVA was adminis- tered as a dietary admixture to male and female Spra- gue–Dawley rats (20/sex/group) at doses of 2000, 3500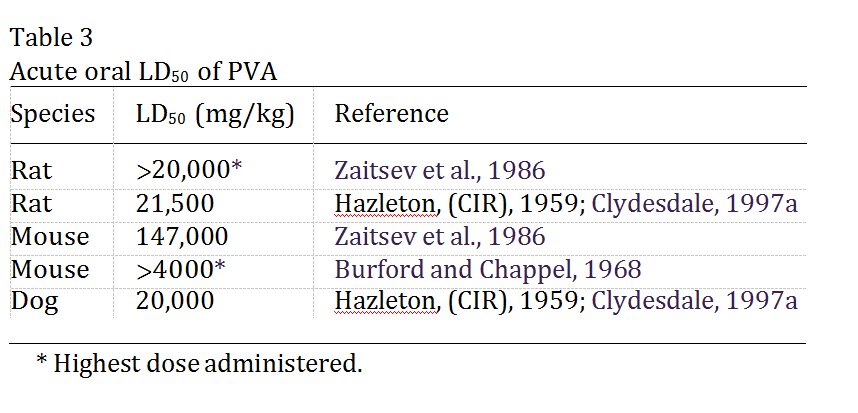

and 5000 mg/kg body weight/day for at least 3 months. A control group (20/sex) received untreated standard laboratory diet (Purina Certified Rodent Diet No. 5002). This study was conducted in compliance with Good Laboratory Practice Regulations of the US FDA, the EEC (99/11/EEC) and the OECD (ENV/MC/ CHEM/(98) 17. Dose levels were selected on the basis of a preliminary 14-day study. The experimental design and implementation are consistent with currently accepted standards (e.g. US FDA, Redbook I and II). Parameters evaluated included twice-daily cageside observations for morbidity, mortality and behavioral effects; weekly body weights and food consumption; periodic hematology, coagulation, clinical chemistry and urinalyses; functional observational battery and motor activity evaluations at selected intervals; oph- thalmoscopic examinations at pretest and prior to sacrifice. All survivors were sacrificed after at least 3 months, selected organs were weighed and organ weight ratios (body, brain) were determined. All animals received complete macroscopic postmortem exami- nations. Selected tissues were examined histopathologi- cally. The only treatment-related effect was unformed stool with anogenital staining in rats receiving 3500 and 5000 mg/kg body weight/day. The no-observed- adverse-effect level (NOAEL) for this study is 5000 mg/ kg body weight/day, the highest dose tested (Colorcon, 2000a).
Carcinogenicity
There are no reported chronic toxicity or carcino- genicity studies of orally administered PVA.
Genotoxicity
PVA was not mutagenic with and without rat liver S9 microsomal activation in Salmonella typhimurium strains TA98, TA100 and TA1537 (Shibuya et al.,1985). Another study which examined the mutagenicity of various dentine bonding agents found that a com- pound containing PVA (of unknown concentration or molecular weight in a mixture of citric acid and ferric chloride) did not induce mutagenic changes in S. typhi- murium strains TA97, TA98, TA100 or TA102 (Schweikl et al., 1996).
Colorcon also conducted a specific set of genotoxi- city studies to provide current supportive information on the specific grade of PVA used as a coating agent for pharmaceutical and dietary supplement products, as well as its potential food use. PVA was evaluated for mutagenicity in histidine-dependent tryptophan auxotrophic mutants of S. typhimurium, strains TA1535, TA1537, TA98 and TA100, and a trypto- phan-dependent mutant of Escherichia coli, strain WP2uvrA/pKM101 (CM 891) in the presence and absence of liver preparations from Aroclor 1254- induced rats (S9 mix). PVA was not mutagenic when tested at concentrations up to 5000 g/plate, the stan- dard limit concentration (Colorcon, 2000b). The potential induction of micronuclei by PVA in bone marrow cells of mice was tested. PVA was administered orally to male and female Swiss mice at doses up to 2000 mg/kg. PVA did not show any evidence of causing chromosome damage or bone marrow cell toxicity at this dose, the standard limit dose (Colorcon, 2000b). PVA was tested for mutagenic potential in an in vitro mammalian cell mutation assay, the detection and quantitation of forward mutation in a subline of mouse lymphoma L5178Y cells, from the heterozygous condi- tion at the thymidine kinase locus (TK +/ ) to the thymidine kinase-deficient genotype (TK / ), in the presence and absence of S9 mix. PVA was not muta- genic in this in vitro cell mutation assay at concentra- tions up to 5000 ı` g/ml, the standard limit concentration (Colorcon, 2000b).
The weight of the evidence indicates that PVA is notmutagenic or clastogenic.
Reproductive toxicity
PVA was administered in the diet to male and female Sprague–Dawley rats (26/sex/group) at doses of 0, 2000, 3500 and 5000 mg/kg body weight/day for two genera- tions. The study design assessed gonadal function, estrous cycle, mating behavior, conception, gestation, parturition, lactation, weaning, and growth and devel- opment of F1 and F2 offspring. Parental rats were trea- ted for 70 days prior to mating, throughout mating, gestation and lactation until sacrifice. Clinical observa- tions, body weights and feed consumption were recor- ded routinely. Dietary concentrations were adjusted for each sex to provide the intended mg/kg/day PVA levels on a weekly basis except during gestation and lactation. Pups were weighed routinely and weaned at 21 days of age prior to selection for the next generation. Unformed stool was noted predominately at the 3500 and 5000 mg/kg body weight/day levels in P0 and F1 parental animals. This finding was attributed to the high levels of PVA being fed and subsequently excreted in the stool. Slight decreases in the mean body weights of P0 males were noted at 2000 and 5000 mg/kg/day. Feed con- sumption was elevated primarily at the 3500 and 5000 mg/kg/day treatment levels in both generations but not during either lactation period. These increases generally were observed in a dose-related manner (g/kg/day), as a result of the large amount of PVA being consumed to maintain the caloric intake necessary for normal growth. There were no effects of PVA on P0 or F1 male or female reproductive performance or pup survival, growth, organ weights, and macroscopic or microscopic observations. Dietary concentrations of 2000, 3500 and 5000 mg/kg/day of PVA to male and female rats did not result in any adverse toxicological or reproductive effects in either parental animals (P0 and F1) or F1 and F2 offspring. The NOAEL for this study was 5000 mg/ kg, the highest dose tested (Colorcon, 2001).
Conclusion
All the available information on PVA gleaned from a comprehensive search of the scientific literature were critically evaluated. Orally administered PVA is rela- tively harmless. The safety of PVA is based on the fol- lowing: (1) the acute oral toxicity of PVA is very low, with LD50s in the range of 15–20 g/kg; (2) orally admi- nistered PVA is very poorly absorbed from the gastro- intestinal tract; (3) PVA does not accumulate in the body when administered orally; (4) PVA is not muta- genic or clastogenic; and (5) NOAELs of orally admi- nistered PVA in male and female rats were 5000 mg/kg body weight/day in the 90-day dietary study and 5000 mg/kg body weight/day in the two-generation reproOrally administered polyvinyl alcohol is safe, and suitable for its intended use as a coating for dietary supplement and pharmaceutical product tablets and capsules. Its food application(s) will be decided by JECFA in June, 2003.
Acknowledgements
The authors thank the Burdock Group, Vero Beach, FL for the comprehensive literature review for PVA. The authors would also like to thank Dr. Joseph Bor- zelleca for assistance in the preparation of the manu- script and for the valuable comments.
Appendix A. Additional studies for routes of exposure other than the oral route
Biological data
Absorption, distribution and excretion
To determine tissue distribution of PVA, female Holtzman rats were injected in the tail vein with 14C- labelled PVA. As noted in Table A1, 64% of the injec- ted dose was excreted in the urine. The hepatic accu- mulation of PVA is probably eliminated into the bile as indicated by the decrease in radiolabelled compound in the liver and an equivalent increase in fecal output of radioactivity (Sanders and Matthews, 1990). However, in a follow-up study, the intravaginal administration of PVA to animals produced varying degrees of accumu- lation within the liver, although in all animals this amount was small (only 2 ppm or 0.2% of total dose after 10 consecutive daily doses of 3 mg/kg body weight) (Sanders and Matthews, 1990).
Hall and Hall (1963) report on the distribution of PVA of low (mol. wt 37,000), medium (mol. wt 133,000) and high (mol. wt 195,000) grades following 4 weeks of daily subcutaneous injection of 1 ml of 5% solutions to female Holtzman rats. Polymers of medium and high molecular weights were found in the tissues of the adrenal medulla, spleen, myocardium, liver and kidney. Low molecular weight polymers were not found in any tissues.
PVA sponges (Ivalon) implanted into guinea pigs (Chen et al., 1969) or rats (dos Santos-Pinto et al., 1969;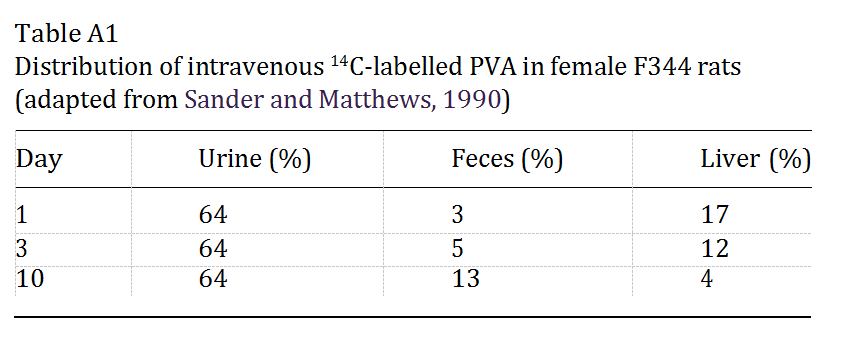
 Holund et al., 1979) appeared to disintegrate or deform, suggestive of phagocytosis by macrophages and giant cells or resorption, but there was no indication of the ultimate fate of the sponges or the cellular PVA.
Holund et al., 1979) appeared to disintegrate or deform, suggestive of phagocytosis by macrophages and giant cells or resorption, but there was no indication of the ultimate fate of the sponges or the cellular PVA.
Therefore it would seem that while subcutaneously injected PVA may undergo bioaccumulation and implanted PVA undergo partial resorption, intravenous or orally administered PVA is quickly eliminated.
Acute toxicity
Finch (1992) reported that the lethality of PVA increases with decreasing percent hydrolysis: the intra- abdominal injection of vinyl alcohol-vinyl acetate copolymers into albino rats resulted in increased mor- tality with increasing molar percent content of vinyl acetate units.
Repeated dosing (subacute) toxicity
Physiological responses are dependent on the mole- cular weight of the administered polymer and the route of administration. Repeated dose studies are reported in Table A2. Hall and Hall (1963) compared the effects of subcutaneously administered PVA of three differing molecular weights (37,000; 133,000; 185,000) in Holtz- man rats over a 4-week period. Daily injections of 1 ml of a 5% solution of low molecular weight PVA elicited no systemic effects but the medium weight PVA induced severe hypertension, abdominal swelling, weight loss, pin-point hemorrhages on the kidneys, cardiac hyper- trophy as well as hepatic, renal and splenic enlargement. High molecular weight PVA induced no, or minimal, extra-renal effects. Although the deposits of medium and high molecular weight PVA in the renal glomeruli were similar, histology showed that there was much less damage caused to the tissue by the higher molecular weight polymer.
Hueper (1939) also reported the results of 15–20 daily subcutaneous injections to rats of 1 cc of a 5% solution of PVA and 10–25 daily intravenous injections of 10–20 cc of 5% PVA to male rabbits. Results were indicative of physiologic reaction to large amounts of injected substance, with mobilization of inflammatory cells (although the PVA tended to remain extracellular),infiltration of substance in endothelial spaces and pool- ing of PVA at the injection sites.2 Although histological examination was extensive, Hueper (1939) only empha- sized the extent to which damage was observed in the kidney, with damage to the endothelium of the glo- merular tuft and tubular degeneration. Morphological changes were also noted in spleen, liver and adrenals. He identified lungs, spleen and testes as being the pri- mary targets following intravenous administration, and kidney, liver and spleen following subcutaneous admin- istration.
Immunologic response to PVA implants
Implants of PVA in humans (Tomashefski et al., 1988) and rats (Holund et al., 1979) initiated a foreign body response with infiltration of mononuclear cells and mild localized inflammatory reactions. The implanta- tion of PVA sponges into the alveolus created by right upper incisor extraction into 24 male Wistar rats showed gradual resorption by macrophages although no foreign-body reaction was detected in the rats by the termination of the experiment after 40 days (dos Santos- Pinto et al., 1969). Subcutaneously implanted PVA sponges (Ivalon) into 20 male Sprague–Dawley rats resulted in the infiltration and formation of granulation tissue within 40 days (Holund et al., 1979). Infiltrating cells included granulocytes and histiocytes and the pre- sence of fibroblasts and marked collagen deposition within the sponge indicates an active fibrotic process which has been found in human PVA (Ivalon) breast implants (Liu and Truong, 1996).
The immunological response is not likely to extend to extra-systemic applications as is demonstrated by two intravaginal studies: the vaginal implantation of PVA sponges (Ivalon) into three New Zealand adult rabbits resulted in little or no pathological effects after 10 days (Chvapil et al., 1979) and only minimal macrophage infiltration of the sponge was observed; the daily intravaginal dosing of 10 ml of 25% PVA (mol. wt 24,000) for 30 days or 2 years into 50 B6C3F1 mice produced no abnormalities compared with similar vehicle control groups receiving only deionized water (NTP, 1998).
Carcinogenicity
Subcutaneous implantation of PVA sponges (Ivalon) in rats was associated with the formation of neoplastic growth. Oppenheimer et al. (1955) reported an incidence of 8.8% malignant tumor development in 25 male Wis- tar rats; Dasler and Milliser (1963) found tumors in nine of the 12 Holtzman rats which survived 18 months post- implant; Walter and Chiaramonte (1965) found tumors in three of 19 animals that survived past 15 months post- implant. Not all reports indicate that tumor development occurred at the site of the implant: Hueper (1959) found Kupffer cell sarcomas and mesotheliomas in 36% of Bethesda black rats that received a single subcutaneous implant of 500 mg PVA powder (mol. wt 20,000 ).
PVA was nominated for testing in the National Tox- icology Program because of its use in medicine and its relatively widespread use in obstetrics to dilate the cer- vix of women prior to initiation of labor (NTP, 1998). Three groups of 100 female B6C3F1 mice were used in the intravaginal study: an untreated control group, a vehicle control group receiving 20 ml deionized water vehicle only, and a dosed group receiving 20 ml 25% PVA in deionized water. The mice were dosed 5 days per week for 104–105 weeks. Survival of dosed mice was similar to that of the two controls. The final mean body weight of vehicle control mice was less than that of the untreated control group. The mean body weights of the dosed mice were less than those of the untreated con- trols from weeks 17 until the end of the study. The only clinical finding was vaginal irritation, observed in six mice in the vehicle control group and 11 mice in the dose group. No neoplasms or non-neoplastic lesions related to with PVA were observed. Lesion incidence in the reproductive tract did not differ in treated vs con- trols. The NTP concluded that ‘‘[u]nder the conditions of this 2-year study, there was no evidence of carcino- genic activity.. .’’ (NTP, 1998).
Observations in humans
The clinical observations of human tissue response to PVA exposure are limited. PVA is used in ophthalmic solutions such as viscid artificial tears. A double-blind study of 5% PVA solution in the treatment of kerato- conjunctivitis resulted in no adverse effects in any of 11 patients (Norn, 1977).
The use of PVA sponge (Ivalon) prosthetic breast implants was abandoned in the 1950s in favor of silicon. Histology of a 40-year-old PVA implant revealed dra- matic structural changes to the prosthesis such as crys- tallization and calcification associated with dense fibrotic tissue and some multinucleated giant cells (Liu and Truong, 1996), probably indicating a chronic low- grade inflammatory response to the foreign material.
PVA injections are still occasionally called for in the therapeutic embolization of abnormal tissues. A mild chronic inflammatory response associated with the for- eign-body response, infiltration of macrophages, and fibrosis were all found at autopsy of three cystic fibrosis patients who died 10–28 months following a bronchial embolization procedure.
There is a single report connecting exposure of PVA to human carcinogenesis. Proutt and Davis (1977) report the case of a 40-year-old man with hemangio- pericytoma of the bladder. This man had a 2-year his- tory of daily dermal exposure to a solution of PVA in water that did not contain solvents or plasticizers.
Other critical reviews
IARC (1979) critically evaluated available informa- tion on PVA administered by various routes and con- cluded that ‘‘Further studies are required before an evaluation can be made of the carcinogenicity of these compounds [vinyl acetate, polyvinyl acetate and poly- vinyl alcohol].’’ The nature of the required studies was not specified in the report. In 1987, IARC placed PVA in Group 3, ‘‘not classified as to (its) carcinogenicity in humans.’’
The Cosmetic Ingredient Review Panel critically eval- uated available information on PVA to determine its safety for use in cosmetic formulations. It was con- cluded that ‘‘Polyvinyl Alcohol is safe as used in cos- metic formulations’’ (CIR, 1998).
References
Burford, R.G., Chappel, C., 1968. Range-finding acute toxicity studies of polyvinyl alcohol, phthalic acid and cellulose acetate phthalate in the mouse. Bio-Research Laboratories, Ltd. Point Claire, Quebec. For: Merck, Sharpe and Dohme Research Laboratories, West Point, PA and Charles E. Frosst & Company, 350 Selby St. Mon- treal, Quebec.
Clydesdale, F.M., 1997a. Food Additives. Toxicology, Regulation and Properties. CRC Press Database (CD-ROM). CRC Press, Boca Raton, FL. (Food Additive Petition (FAP) 6A1963).
Clydesdale, F.M., 1997b. Food Additives. Toxicology, regulation and properties. CRC Press Database (CD-ROM). CRC Press, Boca Raton, FL. (FAP 7B2055, study performed in 1942).
Clydesdale, F.M., 1997c. Food Additives. Toxicology, regulation and properties. CRC Press Database (CD-ROM). CRC Press, Boca Raton, FL. (FAP 7B2055, study performed in 1937).
Colorcon, 2000a. 3-Month PVA Dietary Toxicity Study in Rats. Colorcon, 2000b. PVA Genotoxicity Studies: Bacterial MutationAssay, Mammalian Cell Mutation Assay, Mouse Micronucleus Test.
Colorcon, 2001. Polyvinyl Alcohol (PVA): Study of the Effects of PVA on Fertility, Early Embryonic Development to Weaning, and the Growth and Development when Administered in the Diet to Rats.
DuPont Chem, 1936. The toxicity and potential dangers of polyvinyl alcohols RH-276 and RH-394 and polyvinyl acetate RH-207-U150 with cover letter dated 101592.
Hodge, H.C., Sterner, J.H., 1949. Title?? American Industrial Hygiene Association Quarterly 10, 4.
Hueper, W.C., 1939. Organic lesions produced by polyvinyl alcohol in rats and rabbits. Archives of Pathology 28, 510–531.Isolyser Company, 1998. Isolyser Company, Inc. 4320 International Boulevard, N.W., Norcross, GA.
JSCI, 1968. Bulletin of Osaka Medical College (JP), 14, 1954 March reported in the 1968 Japanese Standards of Cosmetic Ingredients (JSCI), first ed.
Sanders, J.M., Matthews, H.B., 1990. Vaginal absorption of polyvinyl alcohol in Fischer 344 rats. Human and Experimental Toxicology 9, 71–77.
Schweikl, H., Schmalz, G., Gottke, C., 1996. Mutagenic activity of various dentine bonding agents. Biomaterials 17, 1451–1456.
Shibuya, T., Tanaka, N., Katoh, M., Matsuda, Y.T., Morita, K., 1985. Mutagenicity testing of ST-film with the Ames test, chromo- some test in vitro and micronucleus test in female mice. Journal of Toxicology Sciences 10, 135–141.
Wade, A., Weller, P.J. (Eds.), 1994. Handbook of Pharmaceutical Excipients. American Pharmaceutical Association, Washington, DC.
Zaitsev, N.A., Skachkowv, I.N., Sechenov, I.M., 1986. Substantiation of hygienic standards for some polymeric compounds in water with the use of gradual standardization. Gigena Sanita 10, 75–76. (Cited in NTP, 1998).Further reading
Chen, R.W., Musser, A.W., Postlethwait, R.W., 1969. Alterations of and tissue reaction to polyvinyl alcohol sponge implants. Surgery 66 (5), 899–906.
Chvapil, M., Chvapil, T.A., Owen, J.A., Kantor, M., Ulreich, J.B., Eskelson, C., 1979. Reaction of vaginal tissue of rabbits to inserted sponges made of various materials. Journal of Biomedical Materials Research 13, 1–13.
Cosmetic Ingredient Review (CIR), 1998. Final report on the safety assessment of polyvinyl alcohol. International Journal of Toxicol- ogy 17 (5), 67–92.
Dasler, W., Milliser, R.V., 1963. Induction of tumors in rats by sub- cutaneous implants of surgical sponges. Experientia 19, 424–426.dos Santos-Pinto, R., Okamoto, T., de Castro, A.L.,
Callestini, E.A., 1969. Implants of polyvinyl alcohol sponge (Ivalon) following tooth extractions. Oral Surgery, Oral Medicine and Oral Pathology 28 (1), 36–41.
Finch, C.A., 1992. Health and toxicity regulations relating to poly- vinyl alcohol. In: Finch, C.A. (Ed.), Polyvinyl Alcohol—Develop- ments. John Wiley & Sons Ltd.
Hall, C.E., Hall, O., 1963. Polyvinyl alcohol nephrosis: relationship ofdegree of polymerization to pathophysiological effects. Proceedings of the Society for Experimental Biology and Medicine 112, 86–91.
Holund, B., Junker, P., Garbarsch, C., Christoffersen, P., Lorenzen, I., 1979. Formation of granulation tissue in subcutaneously implanted sponges in rats. Acta Pathologica Microbiologica Scandinavica 87 (sect. A), 367–374.
Hueper, W.C., 1959. Carcinogenic studies on water-soluble and inso- luble macromolecules. A.M.A. Archives of Pathology 67, 589–617.
Hueper, W.C., 1939. Organic lesions produced by polyvinyl alcohol in rats and rabbits. Archives of Pathology 28, 510–531.
IARC, 1987. IARC Monographs on the Evaluation of Carcinogenic risks to Humans. Overall Evaluations of Carcinogenicity: An Updating of IARC Monographs Vols. 1–42. Supplement 7. Inter- national Agency for Research on Cancer, Lyon. p. 32.
Liu, L.W., Truong, L.D., 1996. Morphologic characterization of polyvinyl sponges (Ivalon) breast prosthesis. Archives or Pathology and Laboratory Medicine 120 (9), 876–878.
National Toxicology Program (NTP), 1998. Toxicology and Carcino- genesis Studies of Polyvinyl Alcohol (Molecular Weight=24,000) (CAS No. 9002-89-5) in female B6C3F1 Mice (Intravaginal studies). Technical Report Series No. 474. NIH Publication No. 98-3964.
U.S. Department of Health and HumanServices, Public Health Service, National Institutes of Health, Research Triangle Park, NC. Norn, M.S., 1977. Treatment of keratoconjunctivitis sicca with liquid paraffin of polyvinyl alcohol in double-blind trials. Acta Ophthalmologica 55, 945–950.
Oppenheimer, B.S., Oppenheimer, E.T., Danishefsky, I., Stout, A.P., Eirich, F.R., 1955. Further studies of polymers as carcinogenic agents in animals. Cancer Research 15 (4), 333–340.
Peck, H.M., Chappel, C.I., McKinney, S.E., Stuart, R.S., 1971. Poly- mer 37 Safety Assessment. Merck, Sharp & Dohme Research Laboratories, West Point, PA.
Prout, M.N., Davis Jr., H.L., 1977. Hemangiopericytoma of the bladder after polyvinyl alcohol exposure. Cancer 39, 1328–1330.
Riviere, J.E., Coppoe, G.L., Carlton, W.W., Hinsman, E.J., 1980. Polyvinyl alcohol toxicosis as a model of glomerulonephritis in beagle dogs. American Journal of Veterinary Research 41 (4), 502– 505.
Sanders, J.M., Matthews, H.B., 1990. Vaginal absorption of polyvinyl alcohol in Fischer 344 rats. Human and Experimental Toxicology 9, 71–77.
Tomashefski Jr., J.F., Cohen, A.M., Doershuk, C.F., 1988. Longterm histopathologic follow-up of bronchial arteries after therapeutic embolization with polyvinyl alcohol (Ivalon) in patients with cystic fibrosis. Human Pathology 19 (5), 555–561.
Walter, J.B., Chiaramonte, L.G., 1965. The tissue responses of the rat to implanted Ivalon, Etheron, and polyfoam plastic sponges. British Journal of Surgery 52 (1), 49–54.