Polyvinyl alcohol nanofiber based three phase wound dressings for sustained wound healing applications
a Bioscience and Textile Technology Department, Shinshu University, 3-15-1 Tokida, Ueda 386-8567, Japan b Department of Textile Engineering, Mehran University of Engineering and Technology, Jamshoro 76062, Pakistan c Division of Gene Research, Center of R
Abdul Wahab Jatoi , Hiroshi Ogasawara , Ick Soo Kim, Qing-Qing Ni,
1 Abstract
Herein we present our research on a novel three phase antibacterial wound dressing prepared from car- bon nanotubes, silver nanoparticles (AgNPs) and polyvinyl alcohol nanofibers. The AgNPs were generated on carbon nanotubes surfaces to synthesize carbon nanotubes-AgNP nanoparticles which were added into the polyvinyl alcohol nanofibers prior to electrospinning to prepare PVA/carbon nanotubes-AgNP composite nanofibers. All characterizations confirmed the three phase nanofiber architecture. Owing to growth of AgNPs on carbon nanotubes surfaces and embedding into PVA nanofibers the wound dressings are proposed for sustained and safer wound healing applications. The antibacterial test results confirmed excellent bactericidal and prolonged bacterial growth inhibition properties of the nanocomposites which suggest their suitability as sustained antibacterial wound dressing biomaterial.
2 Introduction
Proper wound management needs careful design and construc- tion of wound dressings for early wound healing, safety and com- fort of the patients. Ideal wound dressings should ensure earlier healing, protection from bacterial infections, wound exudate absorbency/transport, cellular regeneration and biological safety via reduced detrimental side-effects. The wound fluid contains nutrients for bacterial growth and provides environmental condi- tions suitable for bacterial colonisations [1,2]. To avoid bacterial infections a suitable antibacterial agent with least detrimental effects and sustained broad spectrum antibacterial properties would be an ideal choice. Silver has excellent broad spectrum antibacterial characteristic including against antibiotic resistant strains. It has effective bactericidal and bacterial growth inhibition potential [3]. However, excess silver release/exposure may induce detrimental effects such as argyria, allergies, carcinoma and may delay the tissue regeneration process and prolong wound healing durations. To exploit excellent antibacterial properties of silver and ensure protection from its detrimental side-effect immobilisa-tion of silver nanoparticles has been proposed [4,5].
Electrospun nanofiber structures owing to their very high sur- face area, higher porosity and resemblance with human extra cel- lular matrix (ECM) have demonstrated several advantages over conventional wound dressings [6]. These structural features of nanofibers promote tissue regeneration, transport wound fluid and ensure breathability for cellular growth and proliferation. Gen-erally nanofibers have surpassed conventional fibers in many applications. Particularly in biomedical field such as in tissue engi- neering [7], antimicrobial [8,9] and drug delivery applications [10], the nano-level properties demonstrated added benefits.
PVA is a biopolymer with very good biocompatibility, biodegradability, fiber formability, chemical resistance, moisture absorbency and swelling characteristics ideal for wound healing applications. PVA nanofibers can absorb wound exudate and sup- port in tissue regeneration owing to their non-toxicity, biocompat- ibility and oxygen permeability [1,11].
Additionally, intermittent wound dressing replacements, longer healing durations and post-recovery issues such as remaining- scars require due attention considering economical aspects and comfort of patients [1]. Thus to prepare biocompatible wound dressings with sustained antibacterial properties a three phase nanofiber structure have been suggested in present research. To ensure biological safety and prolonged antibacterial activities, AgNPs were immobilized on carbon nanotubes and subsequently embedded into the PVA nanofibers. This nanocomposite architec- ture will also enable to avoid intermittent dressing replacement problems. The excellent bactericidal properties and growth inhibi- tion of E. coli and S. aureus strains in LB medium for the tested time (72 h) suggests suitability of the nanocomposite as biocompatible, sustainable and safer wound dressing material.
3 Experimental
3.1 Materials
The polyvinyl alcohol (Mw: 22000) by MP Biomedicals, France was used in preparation of the nanocomposite. The carbon nan- otubes (40–70 nm), nitric acid, sulphuric acid, DMF, acetone, silver nitrate and de-ionized water were obtained from Wako Pure Chemicals, Japan. The agar and Millers LB broth powders were pur- chased from Sigma Aldrich, Japan. Bacterial strains S. aureus (NBRC:12732) and E. coli (NBRC: 3301) were received from NITE Biological Resource Center, Japan.
3.2 Methodology
The carbon nanotubes-AgNP nanoparticles were synthesized by oxidation of pristine carbon nanotubes, nucleation of silver ions
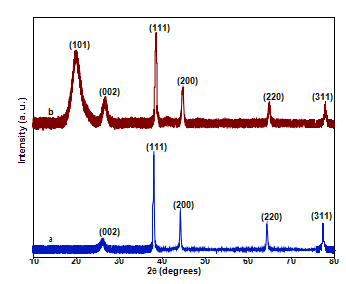
Fig. 1. XRD patterns of (a) carbon nanotube-AgNP nanoparticles (b) PVA/carbon nanotube-AgNP.
and thermal reduction process to synthesize silver nanoparticles (AgNPs). For oxidation, 1 g of carbon nanotubes was added into 200 mL nitric acid and sulphuric acid (1:3) and sonicated for three hours. The carbon nanotubes were collected by centrifugation and dried in a drying oven. For adsorption of silver ions 0.6g of oxidized carbon nanotubes were added in 200 mL of de-ionized water and sonicated for one hour. The carbon nanotubes dispersion was then added into 250 mM silver nitrate solution (200 mL) and kept under strong stirring for three hours. The carbon nanotubes-Ag+ were col- lected by centrifugation at 5000 rpm. In the end the silver nanopar- ticles were synthesized on the carbon bnanotubes (carbon nanotubes-AgNP) by a thermal treatment at 200 °C for two hours.
To prepare PVA based three phase composite nanofibers (PVA/-carbon nanotube-AgNP), the PVA was dissolved in H2O (12 wt%). For complete dissolution the solution was heated at 70 °C for one hour under mild stirring and then kept for about 12 h on magnetic stirrer. Then the carbon nanotubes-AgNP nanoparticles, 5 wt% (car-bon nanotubes-AgNP1) and 10 wt% (carbon nanotubes-AgNP2) were added into the PVA solution and kept under stirring. After- wards, the PVA/carbon nanotubes-AgNP solution was sonicated for 1 h. The PVA/carbon nanotubes-AgNP nanofiber mats were prepared using Katotech Electrospinning Unit (Katotech Co., Japan). Electric voltage used to fabricate nanofibers was 15 kV and dis- tance between tip of needle and a grounded collector was 15 cm.
3.3 Characterizations
The crystallographic studies were conducted by XRD, Mini- Flex300, Rigaku Co., Japan. The TEM images were taken using TEM, JEM-2100, Jeol, USA. For SEM analysis SEM, SU-1510, Hitachi High Technologies was utilized.
The liquid medium antibacterial activities were conducted by incubating samples in LB medium (500 mL) containing 50 mL of 102 serial dilutions of overnight grown E. coli and S. aureus strains. At pre-specified time intervals absorbance (OD590nm) values were taken.
Quantity antibacterial properties were measured by counting the viable cells (CFUs/mL) of the nanofibers relative to CFU/mL of the culture medium without samples. The 108 serial dilutions of the overnight cultured E. coli and S. aureus strains (100 mL) were spread on the agar plates and incubated for 24 h at 37 °C. At the end the CFUs were counted. The relative cell viability (%) was cal- culated as ration of CFUs with nanofibers to CFUs formed without nanofibers.
4 Results and discussions
XRD patterns of carbon nanotube-AgNP and PVA/carbon nanotube-AgNP nanocomposites given in Fig. 1 depict carbon nan- otubes pattern at diffraction angle (2h) 26.250 which refers the (0 0 2) crystal planes [12]. The 2h values at 38.060, 44.20, 64.40, 77.460 correspond to (1 1 1), (2 0 0), (2 2 0) and (3 1 1) crystal.
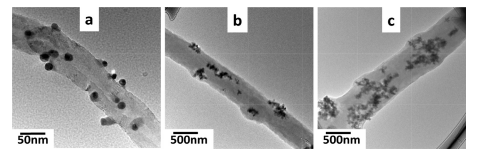
Fig. 2. TEM images of (a) carbon nanotube-AgNP nanoparticles (b) PVA/carbon nanotube-AgNP1 and (c) PVA/carbon nanotube-AgNP2 nanofibers.
planes of metallic AgNPs with FCC structure [13]. PVA pattern can be observed at 19.90 (2h) corresponding to (1 0 1) planes.
Synthesis of AgNPs, their distribution on carbon nanotubes and loading into PVA nanofibers was further studied by TEM observa- tions. The Fig. 2 depicts AgNPs anchored on carbon nanotubes (Fig. 2a) and loaded into the PVA nanofibers (Fig. 2b and c). Owing to the higher concentration of carbon nanotubes/AgNPs (10 wt%) into PVA, the sample PVA/carbon nanotubes-AgNP2 shows the nanoparticles concentrated in the PVA nanofiber matrix.
Morphologies of the nanofibers were observed by SEM studies. The results given in Fig. 3 show regular and bead free PVA, PVA/- carbon nanotubes-AgNP1 and PVA/carbon nanotubes-AgNP2 nano- fibers. May be due to nanoparticles embedding in the nanofiber matrix, their diameters were observed to moderately increase.
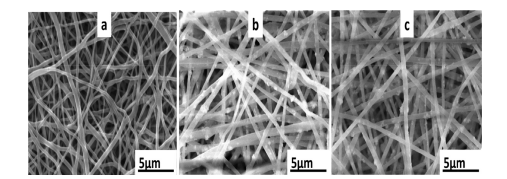
Fig. 3. SEM images of the nanofibers (a) PVA (b) PVA/carbon nanotube-AgNP1 (c) PVA/carbon nanotube-AgNP2.
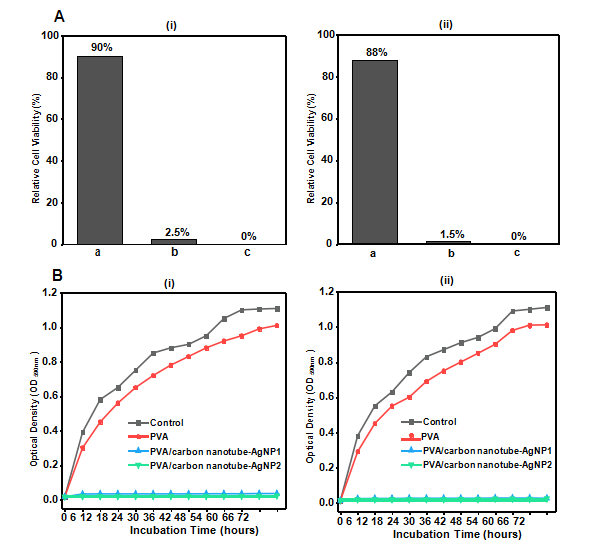
Fig. 4. Antibacterial results of samples (A) relative cell viability (%), against (i) E. coli (ii) S. aureus (a) PVA (b) PVA/carbon nanotubes-AgNP1 (c) PVA/carbon nanotubes-AgNP2 (B) Antibacterial activities, optical density (OD590nm) of the samples against (i) E. coli (ii) S. aureus.
For wound healing the dressings need antibacterial characteris- tics for early curing and protection from infectious bacteria. The quantitative bactericidal properties (relative cell viabilities, Fig. 4A) and growth inhibition of bacteria(optical densities, OD590nm, Fig. 4B) of the PVA/carbon nanotubes-AgNP samples demonstrated the three phase nanofiber system were effective in killing both the test strains and inhibit their growth for the tested time (72 h). The PVA/carbon nanotubes-AgNP2 sample prepared with 10 wt% of carbon nanotube-AgNP nanoparticles yielded 0% viable cells. The PVA/carbon nanotubes-AgNP1 samples exhibited 2.5% and 1.5% relative viable E. coli and S. aureus cells respectively and were equally efficient in growth inhibition of both the strains. Immobilizing the AgNPs on carbon nanotubes as anchoring sub- strate and their localization inside the nanofiber structure would ensure protection from detrimental side effects of silver nanoparti- cles induced by excess silver release/exposure to silver [5]. The proposed mechanism of antibacterial properties of the three phase PVA nanofiber wound dressings may be generation of reactive oxy- gen species (ROS).
5 Conclusions
The research successfully concluded on preparation of a three phase composite nanofibers composed of carbon nanotubes, AgNP and PVA nanofibers for sustained antibacterial activities. The XRD and TEM confirmed synthesis of the carbon nanotubes-AgNP nanoparticles and their incorporation into PVA nanofiber matrix. SEM analysis confirmed regular and bead free nanofiber morpholo- gies. The antibacterial activity tests confirmed extended time antibacterial properties which will help in avoiding frequent replacement of the dressings.
6 References