PVA hydrogel properties for biomedical application
Centre for Advanced Mechanisms and Robotics-CAMAR, School of Mechanical Engineering, Tianjin University, Tianjin, 300072, China
ARTICLEINFO
Article history:
Received 24 January 2011 Received in revised form 1 April 2011
Accepted 6 April 2011
Published online 19 April 2011
Keywords:
Polyvinyl alcohol (PVA) hydrogel Micro-structure
Deformation Mechanical properties Soft tissue
Liver
ABSTRACT
PVA has been proposed as a promising biomaterial suitable for tissue mimicking, vascular cell culturing and vascular implanting. In this research, a kind of transparent PVA hydrogel has been investigated in order to mimic the creatural soft tissue deformation during mini- invasive surgery with needle intervention, such as brachytherapy. Three kinds of samples with the same composition of 3 g PVA, 17 g de-ionized water, 80 g dimethyl-sulfoxide but different freeze/thaw cycles have been prepared. In order to investigate the structure and properties of polyvinyl alcohol hydrogel, micro-structure, mechanical property and deformation measurement have been conducted. As the SEM image comparison results show, with the increase of freeze/thaw cycles, PVA hydrogel revealed the similar micro- structure to porcine liver tissue. With uniaxial tensile strength test, the above composition with a five freeze/thaw cycle sample resulted in Young’s modulus similar to that of porcine liver’s property. Through the comparison of needle insertion deformation experiment and the clinical experiment during brachytherapy, results show that the PVA hydrogel had the same deformation property as prostate tissue. These transparent hydrogel phantom materials can be suitable soft tissue substitutes in needle intervention precision or pre-operation planning studies, particularly in the cases of mimicking creatural tissue deformation and analysing video camera images.
⃝c 2011 Elsevier Ltd. All rights reserved.
Introduction
Polyvinyl Alcohol (PVA), a hydrophilic, biodegradable and biocompatible synthetic polymer, has been widely used in different areas of the biomedical field (Paradossi et al., 2003). Recently, PVA hydrogels have become especially attractive to the field of ‘tissue engineering’ for repairing and regenerating a wide variety of tissues and organs (Woerly et al., 1996), (Hubbell, 1998), including arterial phantom (Chu and Rutt, 1997), heart valves (Jiang et al., 2004), corneal implants (Vijayasekaran et al., 1998), and cartilage tissue substitutes (Stammen et al., 2001).
In tissue engineering, PVA based scaffold has been studied to substitute the current available artificial grafts. Vrana focuses their investigation on the evaluation of the response of the vascular cells to the changes in the hydrogel structure by increasing freeze–thaw cycle number (Vrana et al., 2008). Hoffman has successfully used PVA hydrogels to cultivate living cells, because such hydrogels have large pores and are capable of degradation (Hoffman, 2002).
Hydrogels are water-swollen crosslinked polymer net- works, which often exhibit characteristics, such as tissue-like elasticity and mechanical strength, and the appearance and feel of PVA hydrogel are similar to those of native arterial( Corresponding address: School of Mechanical Engineering, Tianjin University, 92 Weijin Road, Nankai District, Tianjin 300072, China.Tel.: +86 22 27890307; fax: +86 22 27890307.E-mail address: shanjiang@tju.edu.cn (S. Jiang).1751-6161/$ - see front matter ⃝c 2011 Elsevier Ltd. All rights reserved. doi:10.1016/j.jmbbm.2011.04.005)tissue (Nuttelman et al., 2001). The mechanical properties of PVA arterial vessels developed by Chu and Rutt are similar to those of porcine aortas (Chu and Rutt, 1997).PVA can be used not only for bioartificial materials but also for phantom materials commonly used in medical research. Forecasting soft tissue deformation by analysing therapeutic interventions and performing minimally invasive surgery simulations may greatly improve the proposed treatment, as well as the accuracy of surgical procedures (Chanthasopeephan et al., 2007). Simulation experiments require the use of tissue-simulating objects that mimic the properties of human or animal tissues. These phantom materials should have similar deformation rates of elasticity as compared with the target tissue, as well as exhibit long- term structural stability, high water content and excellent transparency.
Using an appropriate ratio of PVA and water, a gel can easily be formed that possesses tissue-mimicking properties. Such a phantom material intended for medical image processing has been introduced by Mano et al. (1986). Likewise, Surry prepared a PVA-containing material with well-described ultrasound and magnetic resonance (MR) characteristics. He found that the velocity of sound in the PVA material was 1520–1540 m/s, which is within the typical range for tissue and is useful for biopsy precision studies (Surry et al., 2002, 2004). T1 values of PVA obtained through MR studies seemed to be similar to values for grey and white matter and muscle. Thus, it was concluded that this material may be appropriate for T1 imaging studies (Bushberg et al., 1994). Gobbi and Peters developed a PVA brain phantom that has been useful in multi-modality research (Gobbi and Peters, 2003).
In order to investigate the biopsy precision, PVA materials are adopted in this research to substitute for real tissues in vitro. This paper presents the preparation of transparent PVA hydrogel material and the evaluation of the effect of freeze/thaw cycle number on the hydrogel structure and mechanical property.
Materials and methods
Preparation of hydrogels
Transparent PVA hydrogel materials were developed in order to quantify PVA phantom deformation by analysis with a video camera in lab. This type of analysis equipment is more readily available and user friendly than CT or MR equipment. During a needle intervention proceeding, a biopsy needle is inserted into the phantom, and a marked target will move during the deformation. To measure the target trajectory, the phantom should be transparent, as well as maintain mechanical properties similar to those of creatural soft tissue. Here, rather than focusing on details of the phantom deformation experiment, we describe the preparation of the transparent PVA materials and the corresponding mechanical results.
It is known that the incorporation of DMSO into water as a solvent will improve the transparency of a material and maintain certain mechanical properties (Hyon et al.,1989). Here, the mixing ratio of the PVA solution to DMSO was maintained at 20/80 by weight, resulting in a PVA hydrogel transparency greater than 90% (Hyon et al., 1989). A homogeneous PVA solution was obtained by heating the PVA mixture, 3 g PVA, 17 g de-ionized water, and the 80 g DMSO solvent at 140 ◦C for 2 h. To form the PVA solution into a solid phantom, the solution was allowed to rest for approximately 20 h in a humid environment, to allow the rise and dissolution of air bubbles at the solution surface. All the solutions were cast into perspex moulds for solidifying.
Each sample was frozen from room temperature to −20 ◦C and maintained at this temperature for 10 h. At the end ofthe freezing stage, the phantom was allowed to thaw to room temperature over 5–9 h, completing one freeze–thaw cycle. Same procedure was repeated for another set of hydrogels 3 times and 5 times respectively. The number of freeze/thaw cycles was varied to evaluate its effect on the micro-structure and mechanical properties of the corresponding PVA sample. The PVA materials were then stored in clean de-ionized water, and the water was replaced regularly to help extend the application life of the phantom material.
Micro-structure comparison
The cross-sectional structure of the PVA hydrogels were examined with a scanning electron microscope-SEM (Hitachi X-650). Before SEM examination, the hydrated hydrogel samples which were 10 mm in thickness and 50 mm in diameter were frozen overnight at −20◦ and then freeze- dried in dry freezone system (Labconco FreeZone 2.5 L) for 20 h. The dehydrated hydrogels were placed in liquid nitrogen for brittle rupture. Therefore, the micro-structure of cross section can be scanned more distinctly. The porcine liver tissue sample of 15 × 15 × 15 mm3 was frozen-dried at the same condition so that its structure could be compared with the PVA hydrogels.
Uniaxial tensile strength test
To determine the relationship between the freeze–thaw cycle number and the mechanical properties of the PVA hydrogels and compared them with porcine liver tissue, uniaxial tests were performed. The shape of the sample was cylindrical with a fixed diameter of 10 mm and a height ranging from 10 mm to 12 mm. It was assumed that there was no change in weight during the experiments, the PVA samples were isotropic and the materials were incompressible. Force and displacement were measured during the loading test by a tensile strength tester (Laizhou LLY-06B). This instrument has a resolution of ±1% and supports loading rates ranging from 0.5 to 60 mm/min. The load cell is able to measure a force of up to 5 N. The temperature was approximately 20 ◦C, and the humidity was maintained between 60%–70% to prevent drying during the procedure. The loading rate was 10 mm/min. The test setup is shown in Fig. 1(a)–(c).
Needle insertion deformation test
Our investigation focuses on the developing of a kind of PVA hydrogel phantom material which can be used in the (
(
Fig. 1 – PVA hydrogel uniaxial tensile test setup. (a) Clamping state. (b) Sample on the tensile strength tester. (c) Porcine liver property test setup.)
soft tissue deformation research during needle intervention. Brachytherapy, an effective treatment for cancer, is a treatment that needs to implant radioactive seeds into people’s organ or around the tumour. Precisely implant of seeds with planned radioactive dose, to irradiate surrounding tissue over several months, will minimize the damage of healthy tissue while maximize the destruction of cancerous cells. However, the force loaded on the tip of insertion needle will cause the tissue deformation during the process and result in the misplaced seeds. This will lead to underdosed regions and complications. As the human tissue is usually unobtainable, with the help of PVA phantom, researcher can investigate the tissue and needle deformation during brachytherapy. This will guide the surgeons to implant the seeds more accurately without touching the important organ or tissue. The insertion experiment setup is shown as Fig. 2(a)–(c). The setup is built up with two high speed cameras (Basler Scount, SCA640-74FM, Sensor pixel is 659 ×494, 74 frame/s) for receiving images during the needle insertion, and with one transparent PVA phantom (edge of the cube is 60 mm), so that the camera can catch the target movement in the phantom. The target is sphere-shape object implanted in advance. The camera caught its original position in the phantom at the beginning of the test. We restricted the hydrogel cube by five planes to complete the boundary conditions. The needle insertion mechanism is an ultrasound motor driving mechanism. The insertion speed is 1 mm/s. Force sensor impedance head (Bruel & Kjaer 8001), charge amplifier (Bruel & Kjaer 2635), and the data acquisition card (NI USB9233) are used to receive the force data during insertion.
1. Results and discussion
1.1. SEM
Three kinds of PVA hydrogel samples were freeze drying before investigation. The SEM images of three kinds of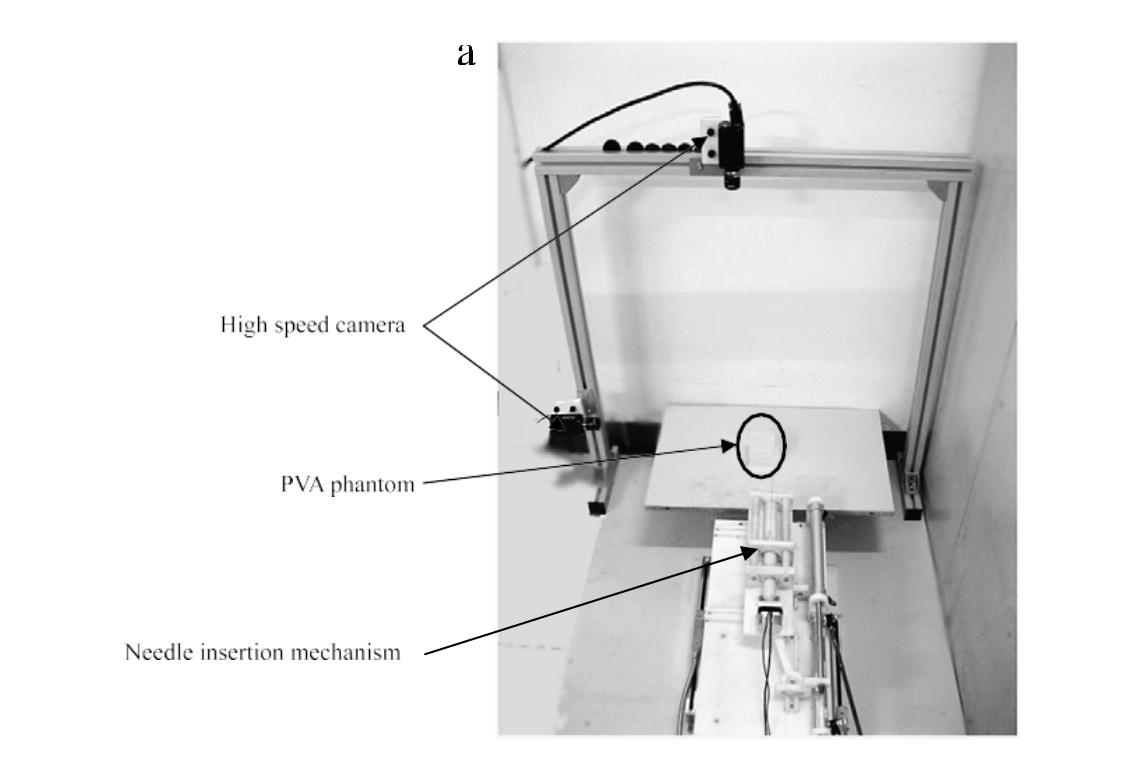


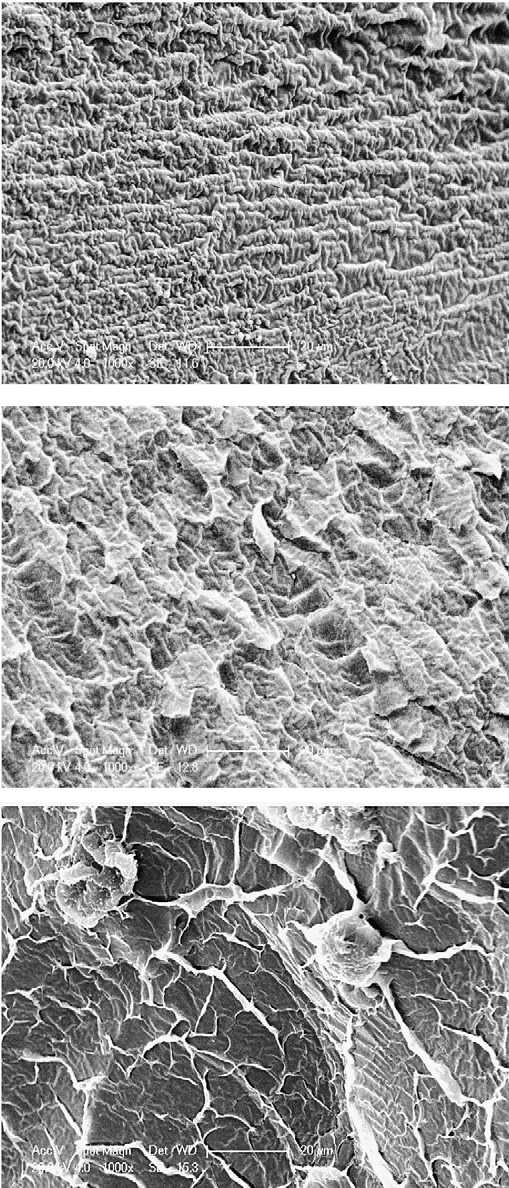
Fig. 3 – PVA SEM images of different freeze–thaw cycles. (a) PVA hydrogel of one freeze–thaw cycle. (b) PVA hydrogel of three freeze–thaw cycles. (c) PVA hydrogel of five freeze–thaw cycles. Fig. 4 – SEM image of porcine liver sample.
Fig. 4 – SEM image of porcine liver sample.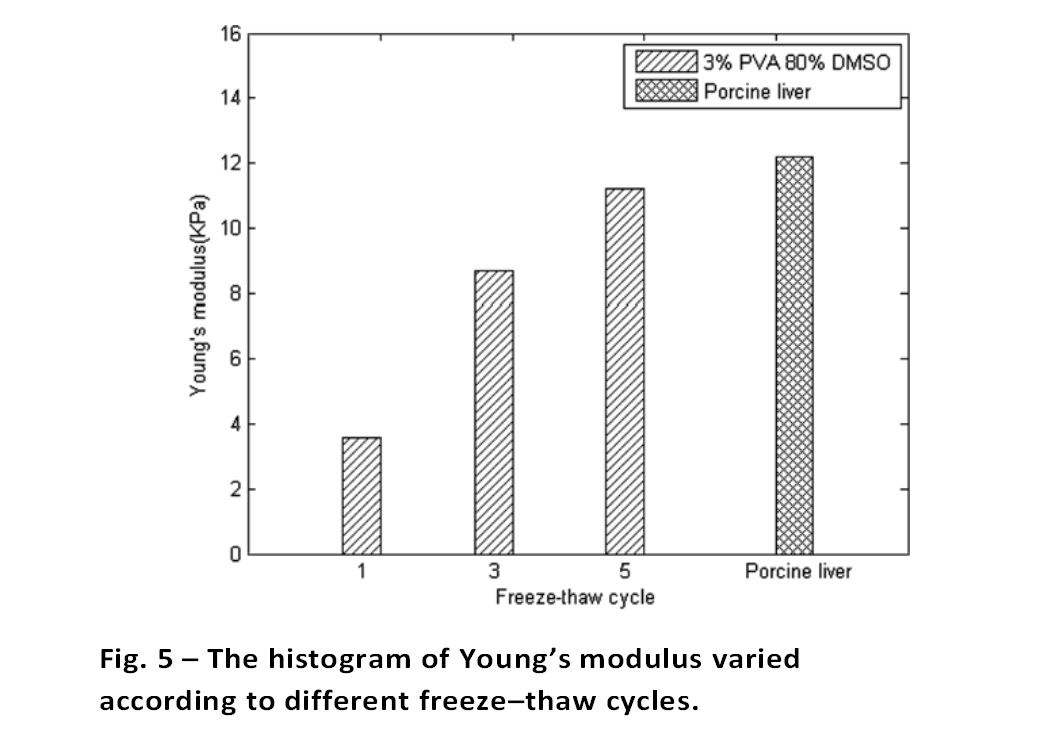 1.1. Uniaxial tensile strength test
1.1. Uniaxial tensile strength test
In this experiment, we evaluated three samples, which were prepared with identical component ratios but varying freeze/thaw cycle repetitions. Samples were solidified in the mould of cylinder shape, 10 mm in length and 10 mm in diameter. The sample was fixed on the clamps by surgical glue.
As shown in Fig. 5, we found that the freeze/thaw cycle number had a substantial effect on the mechanical properties of the PVA materials. Young’s modulus increased from 3.6 kPa to 11.4 kPa, which is almost the same as porcine liver tissues. To exhibit equal strain levels, samples prepared with increasing repetitions of freeze/thaw cycles required increasing levels of applied stress.
1.2. Needle insertion deformation test
The deformation properties of this PVA hydrogel phantom can be partially proved by the quantity measurement during clinical brachytherapy surgery. The relationship between 3D points in phantom and the image plane of camera in the calibration of photographic figure by using Direct Linear Transformation (DLT) is solved. As Stone et al. mentioned in their paper, prostate deformations along the x and y axes referred to the change in the maximum dimension of the gland at the plane through the axis were under consideration. The mean deformations in x and y directions is 4.3–8.1 mm, and 1.0–5.5 mm respectively (Stone et al., 2002). Pre-needle and post-needle contours at a particular plane projected along the x and y axes are used to test the change in length. The deformations measured in x and y directions during insertion is 4.5 mm and 3.27 mm respectively. Fig. 6 shows the force profile loaded on the interactive surface. In this figure, the figure above is the experimental result reported in reference (Podder et al., 2006), at about 92 mm of penetration, needle hits the prostate capsule. The maximum force inside the prostate is 6.21 N. The test result in our research is in the figure below. The maximum force tested is 5.3 N, which is lower than the result reported in references. Since there is no capsulate outside, needle inserted the phantom directly, without puncturing perineum skin as clinical does.This figure shows a resemblance to the force measured in reference.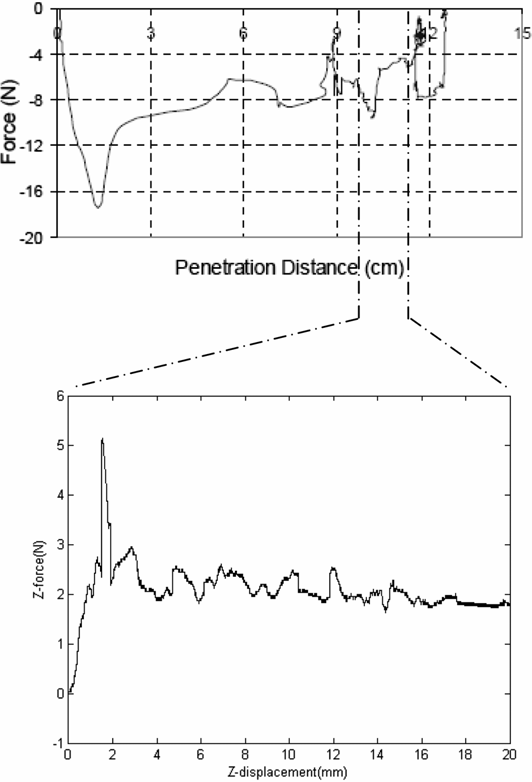
Fig. 6 – Comparison of interactive force load in clinical experiment and on PVA hydrogel during insertion lab experiment. Horizontal axis is the needle displacement during insertion. Vertical axis is the interactive force on z direction.
1. Conclusions
This preparation of PVA material may be a suitable substi- tute for creatural soft tissue in investigations of soft tissue deformation for biopsy precision research. Notably, for ex- periments that use video cameras to observe PVA deforma- tion, transparent PVA materials are required. By varying the freeze/thaw cycles of PVA hydrogel preparations, transpar- ent PVA phantom is developed, which exhibits similar me- chanical properties and morphological characteristics to that of porcine liver, a reference material for human soft tissue. In addition, the number of freeze/thaw cycles of PVA had a marked effect on the stress–strain relationship of PVA hydro- gel and its morphological structure. Moreover, the PVA defor- mation during the video based deformation experiment has the same status as measured during clinical surgery. By main- taining appropriate mechanical properties and transparency, such PVA hydrogels may be suitable substitutions for creatu- ral soft tissues in biopsy precision research.
Acknowledgments
The fund for this project was provided by the National Natural Science Foundation of People’s Republic of China,grant No. 60703045 and also the project was sponsored by the Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry.
REFERENCES
Bushberg, J.T., Seibert, J.A., Leidholt, E.M., Boone, J.M., 1994. The Essential Physics of Medical Imaging. Lippincott Williams and Wilkins, Philadelphia.
Chanthasopeephan, T., Desai, J.P., Lau, A.C.W., 2007. Modelingsoft-tissue deformation prior to cutting for surgical simula- tion: finite element analysis and study of cutting parameters. IEEE Trans. Biomed. Eng. 54, 349–359.
Chu, K.C., Rutt, B.K., 1997. Polyvinyl alcohol cryogel: an ideal phantom material for MR studies of arterial flow and elasticity. Magn. Reson. Med. 37, 314–319.
Gobbi, D.G., Peters, T.M., 2003. Generalized 3D nonlinear transformations for medical imaging: an object-oriented implementation in VTK. Comput. Med. Imaging Graph. 27, 255–265.
Hoffman, A.S., 2002. Hydrogels for biomedical applications. Adv.Drug Delivery Rev. 43, 3–12.
Hubbell, J.A., 1998. Synthetic biodegradable polymers for tissue engineering and drug delivery. Curr. Opin. Solid State Mater. Sci. 3, 246–251.
Hyon, S.H., Cha, W.I., Ikada, Y., 1989. Preparation of transparentpoly hydrogel. Polym. Bull. 22, 119–122.
Jiang, H., Campbell, G., Boughner, D., Wan, W.K., Quantz, M., 2004. Design and manufacture of a polyvinyl alcohol (PVA) cryogel tri-leaflet heart valve prosthesis. Med. Eng. Phys. 26, 269–277.
Mano, I., Goshima, H., Nambu, M., Iio, M., 1986. New polyvinyl alcohol gel material for MRI phantoms. Magn. Reson. Med. 3, 921–926.
Nuttelman, C.R., Mortisen, D.J., Henry, S.M., Anseth, K.S., 2001.Attachment of fibronectin to poly (vinyl alcohol) hydrogels promotes NIH3T3 cell adhesion, proliferation, and migration.
J. Biomed. Mater. Res. B 57, 217–223.
Paradossi, G., Cavalieri, F., Chiessi, E., Spagnoli, C., Cowan, M.K., 2003. Poly(vinyl alcohol) as versatile biomaterial for potential biomedical applications. J. Mater. Sci., Mater. Med. 14, 687–691.
Podder, T.K., Sherman, J., Fuller, D., Messing, E., Rubens, D., Strang,
J., Brasacchio, R., Yu, Y., 2006. In vivo measurement of surgical needle intervention parameters: a pilot study.. Conf. Proc. IEEE Eng. Med. Biol. Soc. 3652–3655.
Stammen, J.A., Williams, S., Ku, D.N., Guldberg, R.E., 2001.
Mechanical properties of a novel PVA hydrogel in shear and unconfined compression. Biomaterials 22, 799–806.
Stone, N.N., Roy, J., Hong, S., et al., 2002. Prostate gland
motion and deformation caused by needle placement during brachytherapy. Brachytherapy 1, 154–160.
Surry, K.J.M., Austin, H.J.B., Fenster, A., Peters, T.M., 2004. Poly
cryogel phantoms for use in ultrasound and MR imaging. Phys. Med. Biol. 49, 5529–5546.
Surry, K.J.M., Smith, W.L., Campbell, L.J., Mills, G., Downey, R.D.B.,
Fenster, A., 2002. The development and evaluation of a three- dimensional ultrasound-guided breast biopsy apparatus. Med. Image Anal. 6, 301–312.
Vijayasekaran, S., Fitton, J.H., Hicks, C.R., Chirila, T.V., Crawford,
G.J., Constable, I.J., 1998. Cell viability and inflammatory response in hydrogel sponges implanted in the rabbit cornea. Biomaterials 19, 2255–2267.
Vrana, N.E., Liu, Y., McGuinness, G.B., Cahill, P.A., 2008.
Characterization of poly(vinyl alcohol)/chitosan hydrogels as vascular tissue engineering scaffolds. Macromol. Symp. 269, 106–110.
Woerly, S., Plant, G.W., Harvey, A.R., 1996. Neural tissue
engineering: from polymer to biohybrid organs. Biomaterials 17 (3), 301–310.